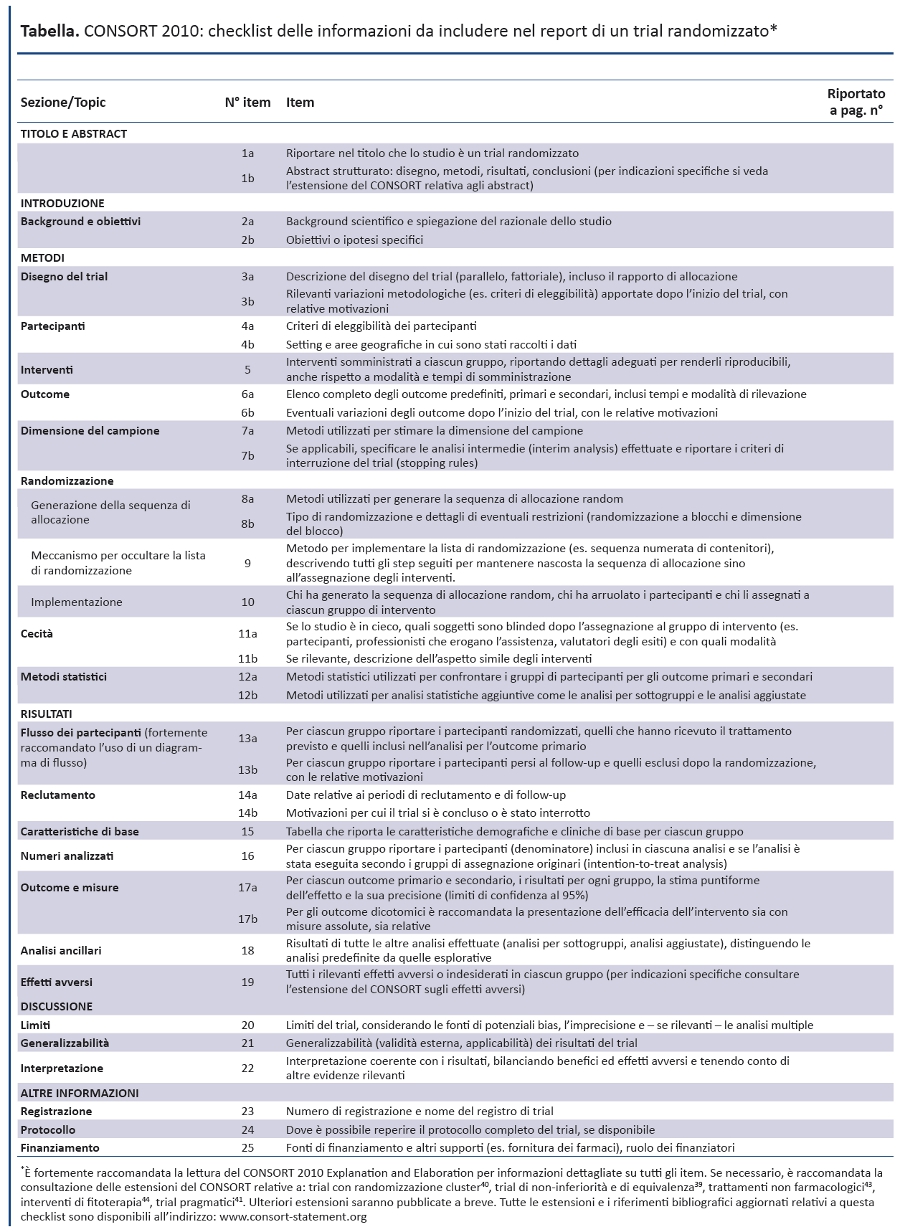
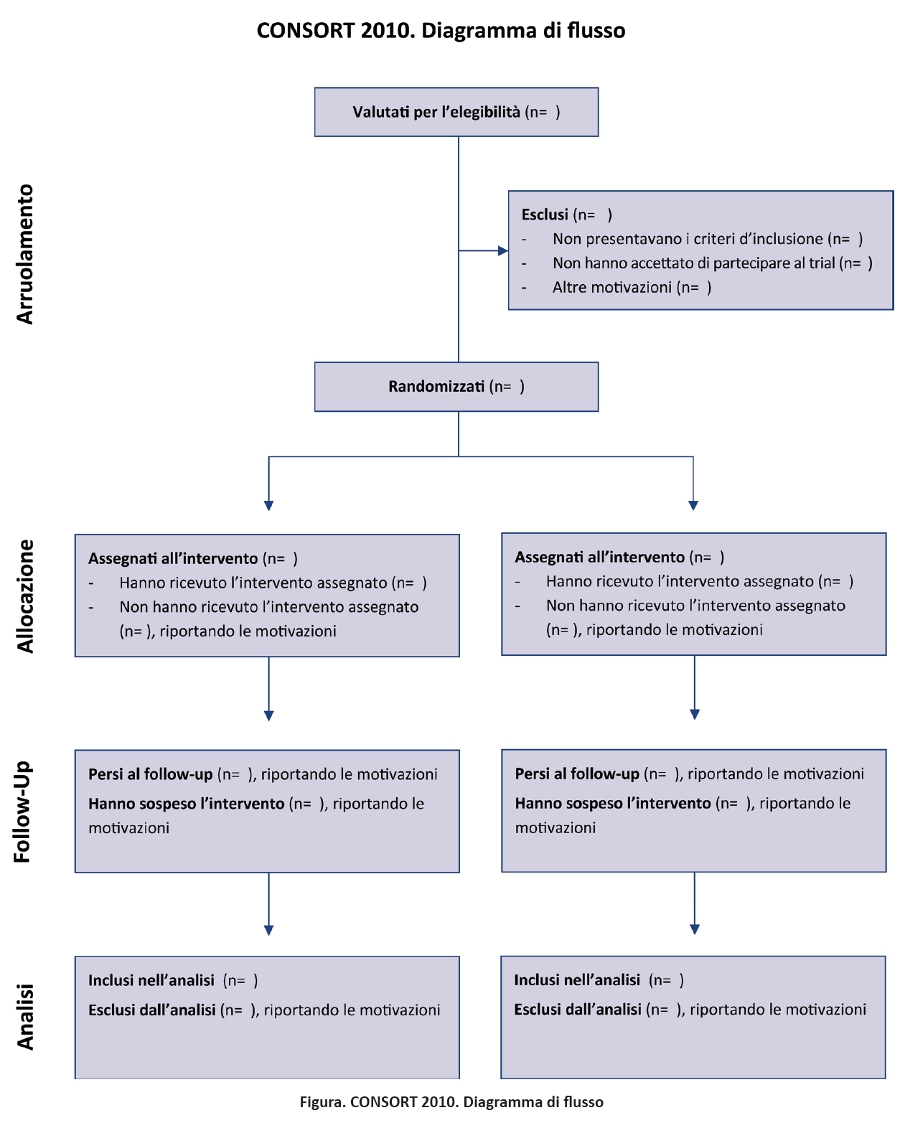
CONSORT Statement 2010: linee guida aggiornate per il reporting di trial randomizzati a gruppi paralleli
Guidelines & Standards
CONSORT Statement 2010: linee guida aggiornate per il reporting di trial randomizzati a gruppi paralleli
Kenneth F Schulz, Douglas G Altman, David Moher, per il CONSORT GroupEvidence 2012;4(7): e1000024 doi: 10.4470/E1000024
Pubblicato: 27 novembre 2012
Copyright: Š 2012 Schulz KF et al. Questo è un articolo open-access, distribuito con licenza Creative Commons Attribution, che ne consente lâutilizzo, la distribuzione e la riproduzione su qualsiasi supporto esclusivamente per fini non commerciali, a condizione di riportare sempre autore e citazione originale.
Vedi anche: CONSORT 2010 Spiegazione ed Elaborazione
1. JĂźni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323:42-6.
2. Chan AW, Altman DG. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet. 2005;365:1159-62.
3. Glasziou P, Meats E, Heneghan C, Shepperd S. What is missing from descriptions of treatment in trials and reviews? BMJ. 2008;336:1472-4.
4. Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan AW, Cronin E, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS One. 2008;3:e3081.
5. Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637-9.
6. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191-4.
7. Moher D, Schulz KF, Altman DG; CONSORT GROUP (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657-62.
8. Moher D, Schulz KF, Altman D; CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987-91.
9. Plint AC, Moher D, Morrison A, Schulz K, Altman DG, Hill C, et al. Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust. 2006;185:263-7.
10. Hopewell S, Dutton S, Yu LM, Chan AW, Altman DG. The quality of reports of randomised trials in 2000 and 2006: a comparative study of articles indexed by PubMed. BMJ. 2010;340:c723.
11. Campbell MK, Elbourne DR, Altman DG; CONSORT group. CONSORT statement: extension to cluster randomised trials. BMJ. 2004;328:702-8.
12. Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ; CONSORT Group. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295:1152-60.
13. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869.
14. Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408-12.
15. Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609-13.
16. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-9, W64.
17. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al; Standards for Reporting of Diagnostic Accuracy. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Intern Med. 2003;138:40-4.
18. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573-7.
19. The Standards of Reporting Trials Group. A proposal for structured reporting of randomized controlled trials. JAMA. 1994;272:1926-31.
20. Rennie D. Reporting randomized controlled trials. An experiment and a call for responses from readers [Editorial]. JAMA. 1995;273:1054-5.
21. Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, et al; CONSORT Group. CONSORT for reporting randomised trials in journal and conference abstracts. Lancet. 2008;371:281-3.
22. Chan AW, Hro´bjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA. 2004;291:2457-65.
23. Sackett DL. Commentary: Measuring the success of blinding in RCTs: donât, must, canât or neednât? Int J Epidemiol. 2007;36:664-5.
24. Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet. 2002;359:696-700.
25. Montori VM, Devereaux PJ, Adhikari NK, Burns KE, Eggert CH, Briel M,et al. Randomized trials stopped early for benefit: a systematic review. JAMA. 2005;294:2203-9.
26. Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ. 1999;319:670-4.
27. Nuovo J, Melnikow J, Chang D. Reporting number needed to treat and absolute risk reduction in randomized controlled trials. JAMA. 2002;287:2813-4.
28. Ioannidis JP, Evans SJ, Gøtzsche PC, OâNeill RT, Altman DG, Schulz K, et al; CONSORT Group. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141:781-8.
29. De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al; International Committee of Medical Journal Editors. Clinical trial registration: a statement from the International Committee of Medical Journal Editors [Editorial]. Ann Intern Med. 2004;141:477-8.
30. Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003;326: 1167-70.
31. Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, et al; CONSORT Group. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med. 2008;5:e20.
32. Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P; CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148: 295-309.
33. Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C; CONSORT Group. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med. 2006;144:364-7.
34. Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al; CONSORT group. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008;337: a2390.
2. Chan AW, Altman DG. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet. 2005;365:1159-62.
3. Glasziou P, Meats E, Heneghan C, Shepperd S. What is missing from descriptions of treatment in trials and reviews? BMJ. 2008;336:1472-4.
4. Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan AW, Cronin E, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS One. 2008;3:e3081.
5. Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637-9.
6. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191-4.
7. Moher D, Schulz KF, Altman DG; CONSORT GROUP (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657-62.
8. Moher D, Schulz KF, Altman D; CONSORT Group (Consolidated Standards of Reporting Trials). The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987-91.
9. Plint AC, Moher D, Morrison A, Schulz K, Altman DG, Hill C, et al. Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust. 2006;185:263-7.
10. Hopewell S, Dutton S, Yu LM, Chan AW, Altman DG. The quality of reports of randomised trials in 2000 and 2006: a comparative study of articles indexed by PubMed. BMJ. 2010;340:c723.
11. Campbell MK, Elbourne DR, Altman DG; CONSORT group. CONSORT statement: extension to cluster randomised trials. BMJ. 2004;328:702-8.
12. Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ; CONSORT Group. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295:1152-60.
13. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869.
14. Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408-12.
15. Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352:609-13.
16. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-9, W64.
17. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al; Standards for Reporting of Diagnostic Accuracy. Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Intern Med. 2003;138:40-4.
18. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573-7.
19. The Standards of Reporting Trials Group. A proposal for structured reporting of randomized controlled trials. JAMA. 1994;272:1926-31.
20. Rennie D. Reporting randomized controlled trials. An experiment and a call for responses from readers [Editorial]. JAMA. 1995;273:1054-5.
21. Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, et al; CONSORT Group. CONSORT for reporting randomised trials in journal and conference abstracts. Lancet. 2008;371:281-3.
22. Chan AW, Hro´bjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA. 2004;291:2457-65.
23. Sackett DL. Commentary: Measuring the success of blinding in RCTs: donât, must, canât or neednât? Int J Epidemiol. 2007;36:664-5.
24. Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet. 2002;359:696-700.
25. Montori VM, Devereaux PJ, Adhikari NK, Burns KE, Eggert CH, Briel M,et al. Randomized trials stopped early for benefit: a systematic review. JAMA. 2005;294:2203-9.
26. Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ. 1999;319:670-4.
27. Nuovo J, Melnikow J, Chang D. Reporting number needed to treat and absolute risk reduction in randomized controlled trials. JAMA. 2002;287:2813-4.
28. Ioannidis JP, Evans SJ, Gøtzsche PC, OâNeill RT, Altman DG, Schulz K, et al; CONSORT Group. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141:781-8.
29. De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al; International Committee of Medical Journal Editors. Clinical trial registration: a statement from the International Committee of Medical Journal Editors [Editorial]. Ann Intern Med. 2004;141:477-8.
30. Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003;326: 1167-70.
31. Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, et al; CONSORT Group. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med. 2008;5:e20.
32. Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P; CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148: 295-309.
33. Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C; CONSORT Group. Reporting randomized, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med. 2006;144:364-7.
34. Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al; CONSORT group. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008;337: a2390.