Il protocollo di un trial clinico riveste un ruolo chiave nella pianificazione, conduzione, interpretazione e revisione esterna dello studio, grazie a una descrizione dettagliata di tutte le procedure pianificate, dall’approvazione etica sino alla disseminazione dei risultati. Un protocollo adeguatamente redatto facilita un’adeguata valutazione degli aspetti scientifici, etici e di sicurezza prima dell’avvio del trial, della sua coerenza e rigore metodologico, oltre che un’adeguata valutazione complessiva della conduzione e dei risultati al termine della sperimentazione clinica. L’importanza dei protocolli è stata enfatizzata da editori (1-6), peer reviewer (7-10), ricercatori (11-15) e da organizzazioni di tutela dei consumatori (16).
A dispetto del loro ruolo fondamentale, una revisione sistematica ha documentato che le linee guida attualmente disponibili per guidare la stesura dei contenuti del protocollo presentano una notevole variabilità di obiettivi e raccomandazioni, spesso non descrivono i metodi con cui sono state sviluppate e raramente riportano un adeguato coinvolgimento degli stakeholders o evidenze scientifiche a supporto delle raccomandazioni (17). Questi limiti dimostrano, in parte, la possibilità di migliorare la qualità dei protocolli che spesso non riportano in maniera adeguata l’outcome primario (25%) (18,19), il metodo di assegnazione al trattamento (54-79%) (20,21), l’utilizzo del blinding (9-34%) (21,22), i metodi per segnalare gli eventi avversi (41%) (23), gli elementi utilizzati per calcolare la dimensione del campione (4-40%) (21,24), le analisi dei dati pianificate (20-77%) (21,24-26), le policy di pubblicazione (7%) (27), il ruolo di sponsor e ricercatori nel disegno dello studio e nell’accesso ai dati (89-100%) (28,29). I problemi alla base di queste carenze del protocollo determinano spesso emendamenti evitabili, scarsa qualità di conduzione del trial e reporting inadeguato in fase di pubblicazione (15,30).
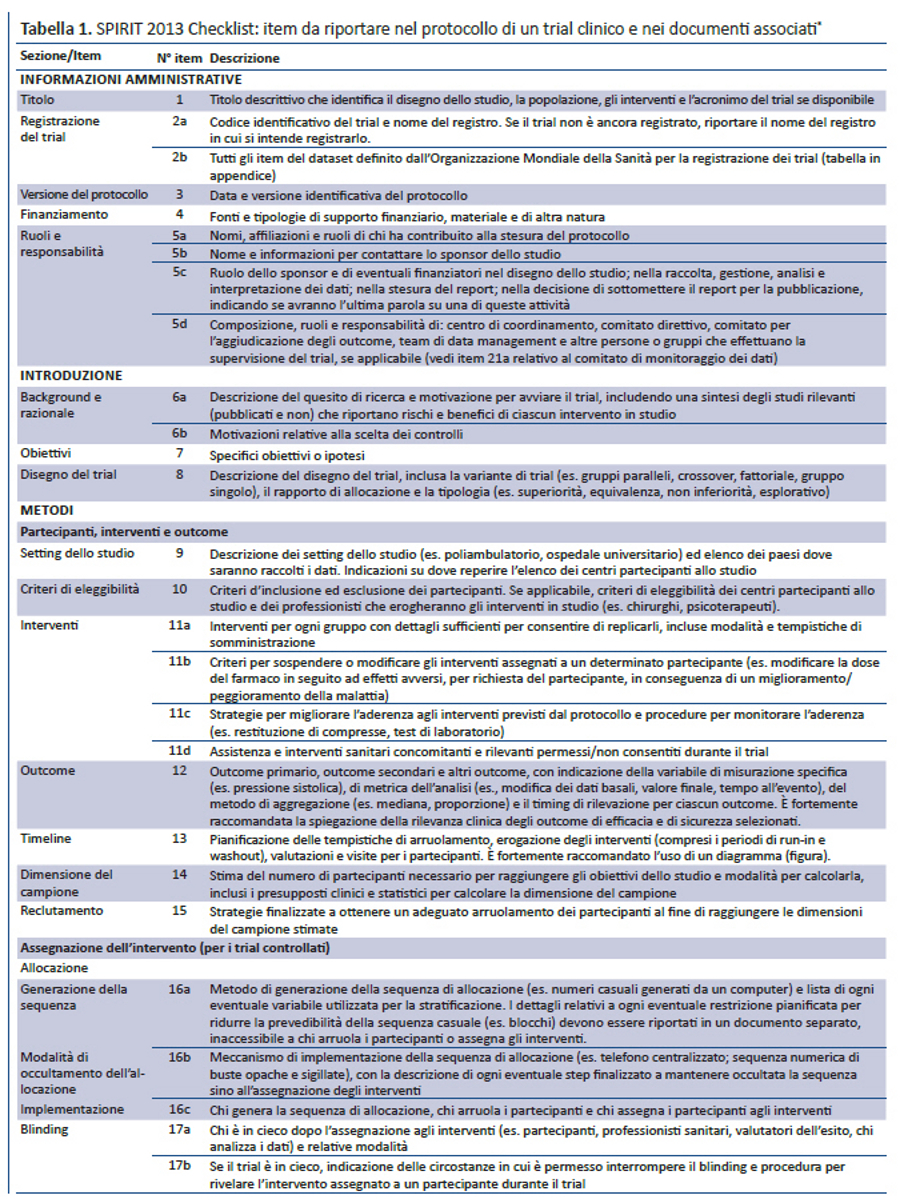
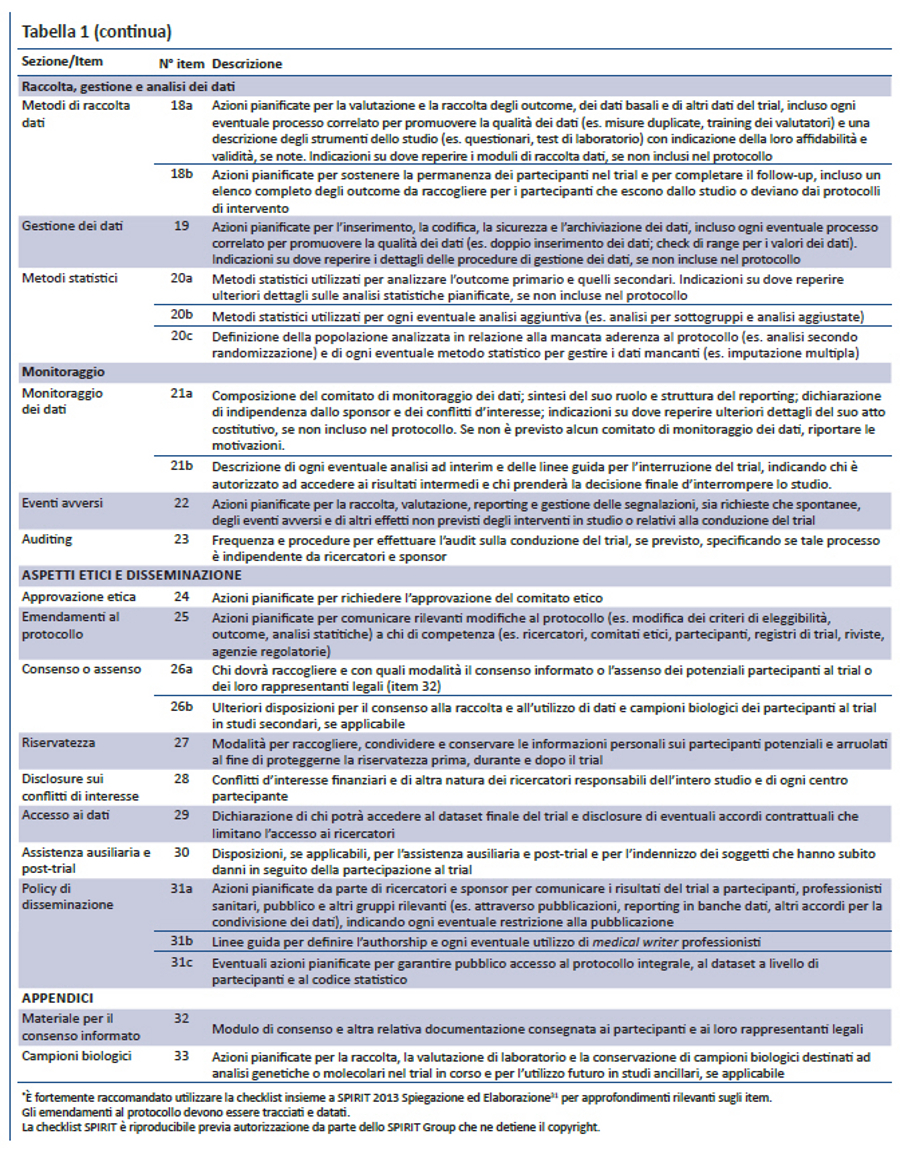
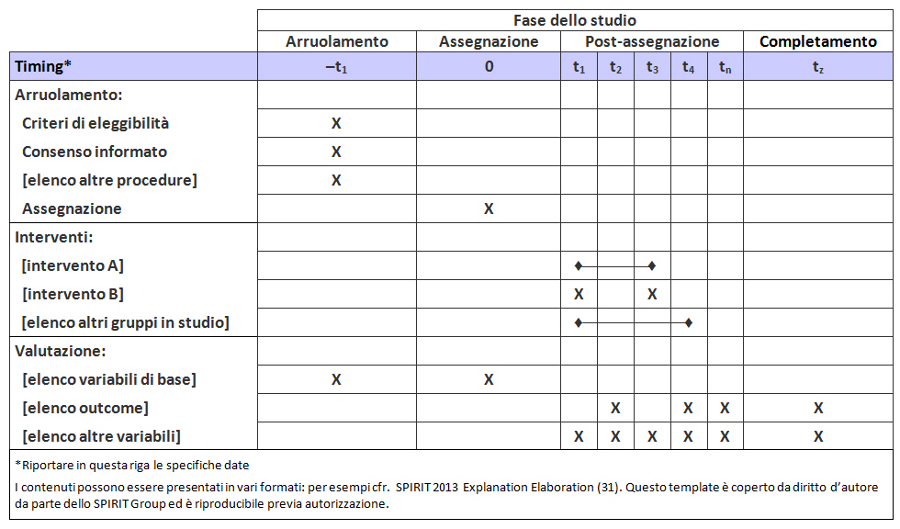
Per colmare questi gap relativi al contenuto dei protocolli dei trial e alle linee guida per la loro redazione, nel 2007 è stata lanciata l’iniziativa SPIRIT — Standard Protocol Item: Recommendations for Interventional Trials (raccomandazioni per definire gli item standard del protocollo dei trial clinici). Questo progetto internazionale ha l’obiettivo di migliorare la completezza dei protocolli dei trial attraverso raccomandazioni evidence-based finalizzate a definire un set minimo di item da includere nei protocolli. Lo SPIRIT Statement 2013 include una checklist di 33 item (tabella 1) e un diagramma (figura). Il documento esplicativo associato (SPIRIT 2013 Spiegazione e Elaborazione) descrive nel dettaglio il razionale e le prove di efficacia a supporto di ciascun item della checklist, fornendo istruzioni e riportando esempi da protocolli reali (31).
SVILUPPO DELLO SPIRIT STATEMENT 2013
Lo SPIRIT Statement 2013 è stato sviluppato attraverso la consultazione di 115 stakeholders con ruoli non mutuamente esclusivi: 30 ricercatori, 31 professionisti sanitari, 34 metodologi, 16 statistici, 14 coordinatori di trial, 15 editori, 17 esperti di etica, 7 sponsor dell’industria e non, 3 agenzie regolatorie. Come descritto in seguito, lo sviluppo dello SPIRIT Statement ha previsto due revisioni sistematiche, un’approvazione formale con il metodo Delphi, due meeting di consenso e un test pilota (32).
La checklist SPIRIT è stata sviluppata in diversi step. Inizialmente è stata definita una checklist preliminare di 59 item, partendo dalla revisione sistematica di recenti linee guida per la redazione di protocolli (17). Nel 2007, 96 esperti rappresentanti di paesi a basso (n= 1), medio (n= 6) e alto reddito (n= 10) hanno perfezionato la checklist preliminare tramite 3 consultazioni via e-mail utilizzando il metodo Delphi (33). I partecipanti hanno valutato la rilevanza di ciascun item assegnando uno score da 1 (non rilevante) a 10 (molto rilevante), hanno suggerito nuovi item e inviato i loro commenti poi condivisi nelle successive consultazioni. Nella consultazione finale gli item con uno score medio = 8 sono stati inclusi nella checklist; quelli con score = 5 sono stati esclusi; gli item con score > 5 e < 8 sono stati oggetto di ulteriore discussione nelle riunioni di consenso.
Dopo le consultazioni con il metodo Delphi, 16 membri del gruppo SPIRIT (gli autori di questo documento) hanno partecipato nel dicembre 2007 a un meeting a Ottawa (Ontario, Canada) e 14 membri a un successivo meeting nel settembre 2009 a Toronto (Ontario, Canada) con l’obiettivo di rivedere i risultati, discutere gli item controversi e rifinire la bozza della checklist. A seguito di ciascun meeting, i componenti del gruppo SPIRIT hanno fornito ulteriori feedback sulla bozza rivista.
Una seconda revisione sistematica ha verificato le evidenze empiriche a supporto di specifici item del protocollo per la conduzione del trial o per il rischio di bias. I risultati di questa revisione sistematica sono stati utilizzati per includere o escludere eventuali item della checklist SPIRIT. Inoltre, la revisione ha fornito le evidenze riportate nell’articolo di Spiegazione ed Elaborazione (31). Alcuni item con pochissime, o addirittura nessuna, evidenze empiriche a supporto (ad es. il titolo) sono stati inclusi nella checklist per la loro rilevanza pragmatica o etica.
Infine, nel 2010 e nel 2011 i laureati dell’Università di Toronto hanno effettuato un test pilota della bozza della checklist, sviluppando protocolli di ricerca durante un master sulla metodologia dei trial clinici. I loro feedback su contenuto, format e utilità della checklist sono stati raccolti con una survey anonima e integrati nella versione definitiva della checklist SPIRIT.
DEFINIZIONE DI “PROTOCOLLO DI UN TRIAL CLINICO”
Sebbene ogni sperimentazione clinica richieda un protocollo, la sua definizione è molto variabile tra ricercatori, sponsor e altri stakeholders. Secondo l’iniziativa SPIRIT il protocollo è definito come un documento che fornisce sufficienti dettagli da garantire la comprensione di: background, razionale, obiettivi, popolazione in studio, interventi, metodi, analisi statistiche, aspetti etici, azioni per la gestione e divulgazione del trial; riproducibilità degli aspetti metodologici rilevanti e di conduzione del trial; valutazione del rigore scientifico ed etico del trial, dall’approvazione etica alla divulgazione dei risultati.
Il protocollo di un trial è molto più di un elenco di item: dovrebbe essere un documento coerente che descrive adeguatamente il contesto e riporta gli elementi utili a garantire la piena comprensione di tutti gli aspetti del trial. Ad esempio, la descrizione di un intervento sanitario complesso può richiedere l’inclusione di materiali e figure esplicative per consentirne la riproducibilità da parte di soggetti con adeguate competenze.
Considerato che il protocollo integrale deve essere sottoposto all’approvazione del comitato etico (34), raccomandiamo a ricercatori e sponsor di verificare la presenza degli item della checklist SPIRIT prima di sottoporlo alla valutazione del comitato etico. Se i dettagli di alcuni item non sono ancora stati definiti, bisognerebbe precisarlo esplicitamente nel protocollo prevedendone un successivo aggiornamento.
Il protocollo è un documento “vivo”, spesso modificato durante il trial. Un audit trasparente, che traccia le date delle modifiche rilevanti nel disegno e nella conduzione del trial, rappresenta una componente essenziale di un lavoro scientifico. Ricercatori e sponsor sono tenuti ad aderire al protocollo, così come approvato dal comitato etico e di documentare gli emendamenti effettuati nella versione più recente del protocollo. Tutti i rilevanti emendamenti al protocollo dovrebbero essere comunicati al comitato etico nel momento in cui vengono apportati, oltre che riportati nei registri di trial e descritti nei report del trial.
SCOPO DELLO SPIRIT STATEMENT 2013
Lo SPIRIT Statement 2013 si applica al contenuto del protocollo di un trial clinico, appendici comprese. Un trial clinico è uno studio prospettico dove uno o più interventi sanitari vengono assegnati ai partecipanti per valutare la loro efficacia su specifici outcome. Lo SPIRIT 2013 si applica in particolare ai trial controllati randomizzati, anche se la checklist può essere utilizzata per tutte le varianti di trial clinici, indipendentemente dal disegno, dall’intervento in studio o dall’area clinico-assistenziale.
Lo SPIRIT Statement 2013 fornisce raccomandazioni per la stesura del dataset minimo di un protocollo: in particolari situazioni può essere necessario integrare il protocollo con ulteriori item. Ad esempio, un trial con disegno fattoriale può richiedere una specifica motivazione; un trial con disegno crossover presenta aspetti statistici molto specifici, come gli effetti carry-over; i trial sponsorizzati dall’industria possono richiedere requisiti regolatori aggiuntivi.
Il protocollo e le sue appendici sono spesso l’unico “contenitore” dove reperire tutte le informazioni rilevanti degli item della checklist SPIRIT. Utilizzando protocolli di trial reali, abbiamo identificato per ciascun item esempi che documentano l’applicabilità di tutti gli item della checklist in un unico protocollo31. In alcuni trial, dettagli rilevanti possono essere riportati in documenti correlati: analisi statistiche pianificate, scheda raccolta dati, manuali operativi, contratti dei ricercatori (35,36). In queste situazioni, il protocollo dovrebbe riportare gli elementi chiave e rimandare ai documenti integrativi per i dettagli.
Lo SPIRIT Statement 2013 si applica soprattutto ai contenuti del protocollo piuttosto che alla forma, spesso condizionata da normative locali, tradizioni o procedure operative standard. Tuttavia, la revisione del protocollo sarà facilitata dall’aderenza ad alcuni standard redazionali: indice, titoli dei capitoli, glossario, lista di abbreviazioni, bibliografia, schema relativo alle fasi di arruolamento, somministrazione degli interventi e valutazione degli outcome (figura).
SPIRIT 2013, infine, ha l’obiettivo di favorire la trasparenza e la completa descrizione di cosa è stato pianificato, ma non ha lo scopo di raccomandare come disegnare o condurre un trial. Di conseguenza, la checklist non dovrebbe essere usata per valutare la qualità dei trial, perché il protocollo di un trial mal disegnato potrebbe riportare tutti gli item della checklist, descrivendo caratteristiche del disegno di studio assolutamente inadeguate. Tuttavia, l’uso di SPIRIT 2013 può migliorare validità e risultati dei trial, in quanto ricorda ai ricercatori quali aspetti considerare durante le fasi di pianificazione dello studio.
RELAZIONI CON ALTRE LINEE GUIDA SUI TRIAL CLINICI
Grazie a un processo di sviluppo sistematico, alla consultazione con stakeholders internazionali e al documento di Spiegazione ed Elaborazione che riporta evidenze rilevanti (31), SPIRIT 2013 prende in considerazione altri documenti internazionali relativi ai protocolli dei trial clinici. Rispetta i principi etici definiti dalla Dichiarazione di Helsinki 2008, in particolare il requisito per cui il protocollo deve affrontare specifiche questioni etiche, come i conflitti di interesse34.
Inoltre, SPIRIT 2013 include gli item raccomandati dalla linea guida E6 della International Conference on Harmonisation Good Clinical Practice, redatta nel 1996 per i trial che devono essere sottoposti agli enti regolatori37. Il documento SPIRIT integra anche la Good Clinical Practice, fornendo raccomandazioni aggiuntive su specifici item chiave del protocollo (es. occultamento dell’assegnazione, registrazione del trial, processi relativi al consenso informato). Diversamente da SPIRIT, la Good Clinical Practice ha utilizzato metodi informali per l’approvazione, ha una contributorship incerta e non riporta evidenze scientifiche a supporto (38).
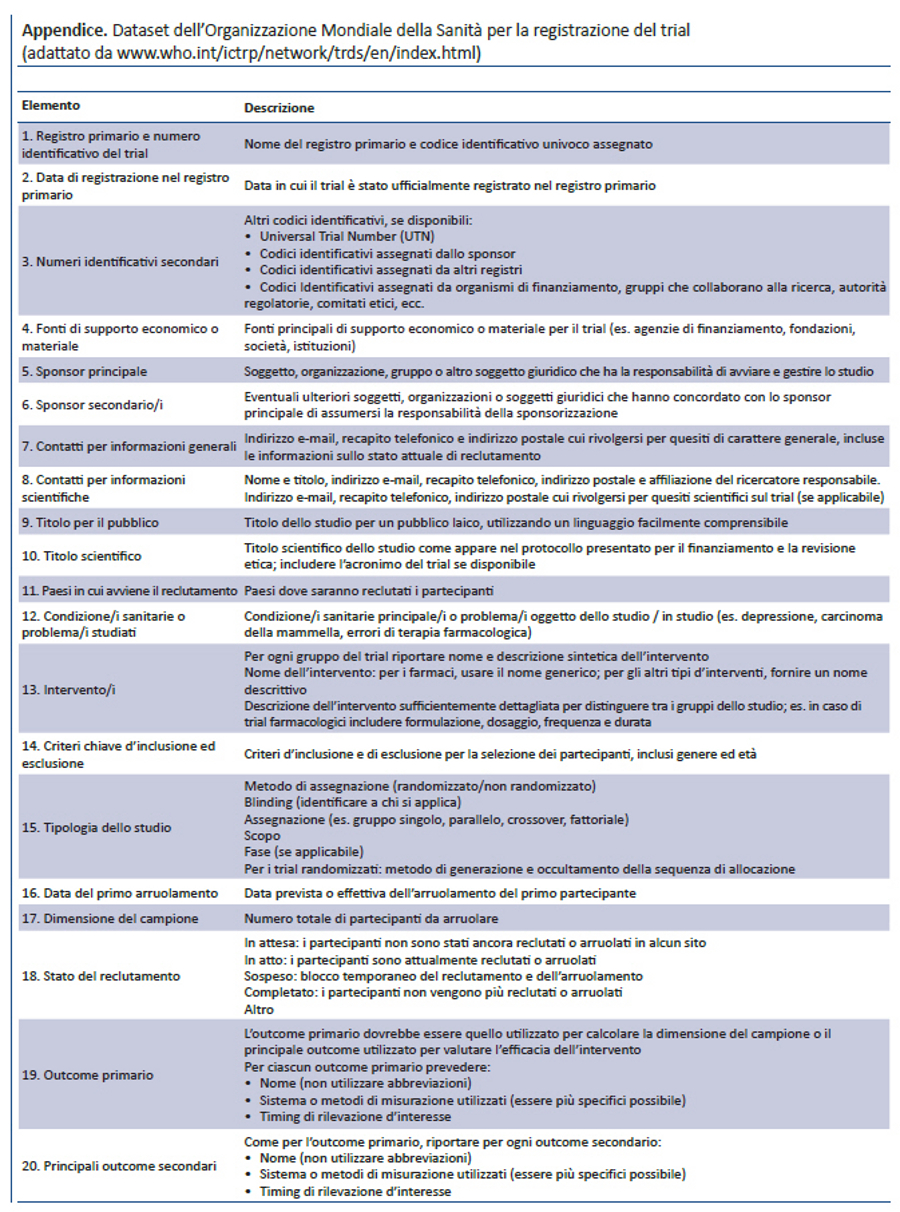
Lo SPIRIT Statement 2013 aderisce anche ai requisiti di registrazione dei trial richiesti dall’Organizzazione Mondiale della Sanità (OMS) (39), a quelli dell’International Committee of Medical Journal Editors (40), alle normative previste da ClinicalTrials.gov (41) e dalla Commissione Europea (42). Ad esempio, l’item 2b della checklist SPIRIT raccomanda che il protocollo riporti il minimum dataset per la registrazione dei trial richiesto dall’OMS (appendice), che l’International Committee of Medical Journal Editors richiede per i registri di trial. Inserire questo dataset nel protocollo non significa fornire solo una buona sintesi del trial, ma aiuta a migliorare la qualità delle informazioni da inserire nei registri. Infatti, i dati richiesti dalla registrazione possono essere facilmente identificati nelle specifiche sezioni del protocollo e riportati nei relativi campi del registro. Inoltre, gli emendamenti al protocollo applicabili a questa sezione potrebbero incoraggiare i ricercatori ad aggiornare i dati del trial nei registri.
Alcuni item dello SPIRIT Statement 2013 sono comuni al CONSORT 2010 (Consolidated Standards of Reporting Trials) (43). L’utilizzo di una terminologia e di una struttura coerenti per gli item comuni alle due checklist faciliteranno il passaggio da un protocollo conforme a SPIRIT a un report finale del trial aderente al CONSORT. Al fine di allineare gli sforzi internazionali finalizzati a promuovere la trasparenza dei trial e la qualità elevata del contenuto dei protocolli, il gruppo SPIRIT ha coinvolto anche i responsabili di altre iniziative rilevanti per gli standard del protocollo: registri di trial, Clinical Data Interchange Standards Consortium Protocol Representation Group, Pragmatic Randomized Controlled Trials in Health-Care.
POTENZIALE IMPATTO
Diversi stakeholders potrebbero trarre beneficio da un utilizzo diffuso dello SPIRIT Statement 2013 e del suo documento esplicativo (tabella 2). Il test pilota e feedback informali hanno dimostrato che SPIRIT è uno strumento particolarmente utile durante la stesura della bozza del protocollo, oltre che un’eccellente risorsa informativa per ricercatori, revisori e componenti di comitati etici. Inoltre, s’intravedono anche effetti positivi sulla conduzione del trial: infatti, l’eccessivo ritardo tra la stesura del protocollo, l’approvazione etica e l’avvio del reclutamento dei partecipanti rappresenta ancora un problema rilevante per i trial clinici (44). Migliorare la completezza del protocollo potrebbe renderne più efficiente la revisione, limitando così le richieste di informazioni su sezioni incomplete o non chiare. Grazie a una documentazione completa delle informazioni chiave e una maggiore consapevolezza degli aspetti rilevanti prima dell’avvio della sperimentazione clinica, SPIRIT può anche contribuire a ridurre il numero e l’impatto dei successivi emendamenti, molti dei quali possono essere evitati con una scrupolosa stesura del protocollo (15). Una diffusa adozione di SPIRIT 2013 come standard univoco da parte di comitati etici, finanziatori istituzionali e sponsor privati, agenzie regolatorie e riviste potrebbe semplificare il lavoro di ricercatori e sponsor, al fine di soddisfare richieste comuni da parte di vari stakeholders con un unico protocollo basato su SPIRIT. Inoltre, una migliore stesura del protocollo potrebbe aiutare tutti i soggetti coinvolti nel trial a condurlo come proposto dagli autori.
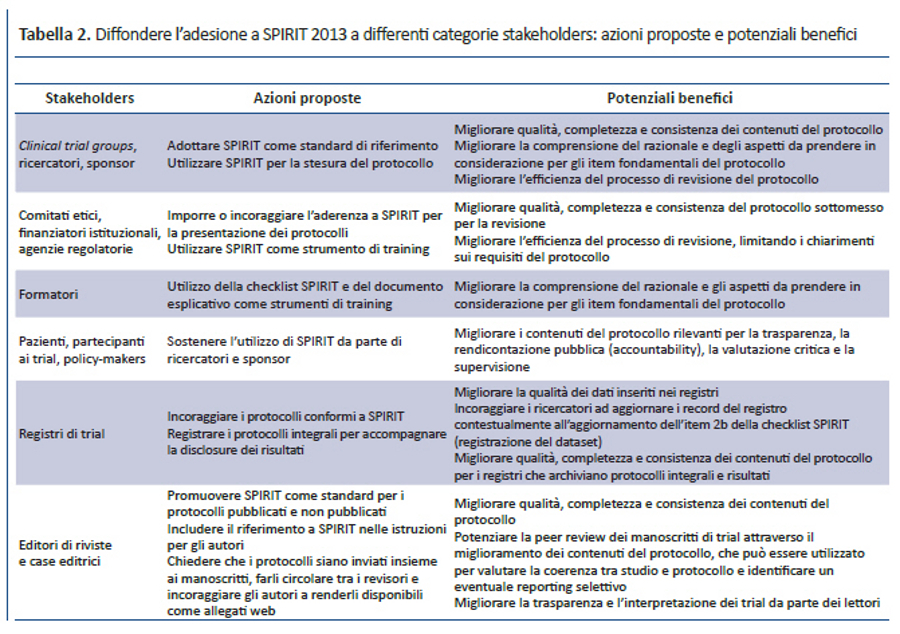
L’aderenza a SPIRIT 2013 permetterebbe di garantire che i protocolli contengano le informazioni necessarie per la valutazione critica e l’interpretazione del trial. Protocolli di elevata qualità possono fornire importanti informazioni sui metodi e sulla conduzione del trial non disponibili sulle riviste o nei registri dei trial (45-47). Considerato che il protocollo riflette in maniera inequivocabile gli intenti originali dei ricercatori, il confronto tra protocolli e report finali dei trial permettono di identificare sia il reporting selettivo degli outcome, sia emendamenti non dichiarati (48), sia eventuali modifiche dell’outcome primario (19,49). L’impatto dello SPIRIT Statement 2013 sarà ancora maggiore quando — a differenza di quanto accade oggi (45) — tutti i protocolli saranno pubblicamente accessibili, consentendo una valutazione completa della validità e applicabilità dei trial clinici (11,12,14,50).
Per avere il massimo impatto, SPIRIT 2013 dovrà essere supportato dai principali stakeholders (tabella 2), così come per altre linee guida per il reporting, come il CONSORT Statement (51). Noi ci impegniamo a pubblicare l’elenco delle organizzazioni che sostengono SPIRIT 2013 sul sito web (www.spirit-statement.org) e a fornire le risorse necessarie per facilitarne l’implementazione. L’adozione diffusa delle raccomandazioni di SPIRIT può migliorare la stesura del protocollo, i contenuti e l’implementazione; facilitare la registrazione, l’efficienza e la valutazione dei trial; in definitiva aumentare la loro trasparenza a beneficio dell’assistenza ai pazienti.