Guidelines & Standards
Evidence 2015;7(6): e1000114 doi: 10.4470/E1000114
Pubblicato: 27 giugno 2015
Copyright: © 2015 Moher et al. Questo è un articolo open-access, distribuito con licenza Creative Commons Attribution, che ne consente l’utilizzo, la distribuzione e la riproduzione su qualsiasi supporto esclusivamente per fini non commerciali, a condizione di riportare sempre autore e citazione originale.
Vedi anche: PRISMA Statement per il reporting di revisioni sistematiche e meta-analisi degli studi che valutano gli interventi sanitari: spiegazione ed elaborazione
La versione italiana del PRISMA Statement è dedicata ad Alessandro Liberati, pioniere nella scienza delle revisioni sistematiche, fermo sostenitore dell’integrità e trasparenza della ricerca e paladino del coinvolgimento dei pazienti nel definire le priorità della ricerca. Alessandro ci ha lasciati il 1 gennaio 2013.
INTRODUZIONE
Revisioni sistematiche e meta-analisi hanno acquisito un ruolo sempre più rilevante per l’assistenza sanitaria: i professionisti sanitari le utilizzano per mantenersi aggiornati (1,2); rappresentano evidenze fondamentali nella produzione di linee guida; gli enti che erogano finanziamenti per la ricerca possono richiedere una revisione sistematica per essere certi che sia necessario avviare ulteriori studi (3) e anche alcune riviste biomediche si stanno muovendo nella stessa direzione (4). Analogamente ad altre tipologie di pubblicazioni, il valore di una revisione sistematica dipende dalle metodologie di conduzione, dalla rilevanza dei risultati e dalla chiarezza del reporting: di conseguenza, la variabile qualità del reporting delle revisioni sistematiche limita la possibilità di valutarne punti di forza e di debolezza.
Diversi studi hanno valutato la qualità dei report delle revisioni sistematiche. Nel 1987 Murlow (5), esaminando 50 review pubblicate nel 1985-86 in quattro prestigiose riviste biomediche, ha rilevato che nessuna soddisfaceva gli 8 criteri definiti, tra cui la valutazione della qualità degli studi inclusi. Nel 1987 Sacks et coll. (6) hanno valutato l’adeguatezza del reporting di 83 meta-analisi, basandosi su 23 caratteristiche organizzate in sei domini: il reporting era di bassa qualità e venivano riportate adeguatamente tra 1 e 14 caratteristiche (media 7.7; deviazione standard 2.7). Nel 1996, un aggiornamento di questo studio ha documentato solo un lieve miglioramento (7).
Nel 1996, nel tentativo di risolvere il reporting non ottimale delle meta-analisi, un gruppo internazionale ha sviluppato la linea guida QUOROM Statement (QUality Of Reporting Of Meta-analyses), per migliorare il reporting delle meta-analisi di trial controllati randomizzati (8). In questo articolo sintetizziamo la revisione di questa linea guida, rinominata PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) e aggiornata per affrontare le diverse novitĂ concettuali e pratiche nella scienza delle revisioni sistematiche (box 1).
|
Box 1. L’evoluzione del QUOROM in PRISMA: aspetti concettuali La conduzione di una revisione sistematica è un processo dinamico. La conduzione di una revisione sistematica dipende in gran parte dall’obiettivo e dalla qualità degli studi inclusi: per tale ragione gli autori potrebbero avere la necessità di modificare il protocollo originale della revisione durante la sua conduzione. Le linee guida per il reporting delle revisioni sistematiche dovrebbero raccomandare di riportare e spiegare tali modifiche, senza farle sembrare inopportune. Il PRISMA Statement (item 5, 11, 16, 23) riconosce la natura dinamica di questo processo. Escludendo le revisioni Cochrane, che prevedono sempre la stesura di un protocollo, solo il 10% dei revisori dichiara di lavorare sulla base di un protocollo (22). Ovviamente, in assenza di un protocollo pubblicamente accessibile è difficile valutare l’appropriatezza di eventuali modifiche. |
TERMINOLOGIA
La terminologia utilizzata per descrivere le revisioni sistematiche e le meta-analisi si è evoluta nel tempo. Una delle ragioni che ha portato a cambiare il nome da QUOROM a PRISMA è stata la volontà di includere oltre alle meta-analisi anche le revisioni sistematiche. Si è scelto di adottare le definizioni della Cochrane Collaboration (9), secondo cui una revisione sistematica, a partire da un quesito formulato in modo chiaro, utilizza metodi espliciti e sistematici per identificare, selezionare e valutare criticamente la ricerca rilevante e per raccogliere e analizzare i dati degli studi inclusi nella revisione. Il termine meta-analisi definisce l’utilizzo di tecniche statistiche per sintetizzare i risultati degli studi inclusi in una revisione sistematica.
SVILUPPO DEL PRISMA STATEMENT
Nel giugno 2005 a Ottawa (Ontario, Canada) si è svolto un meeting di tre giorni, al quale ha partecipato un gruppo di 29 persone, tra cui autori di revisioni sistematiche, metodologi, clinici, editori di riviste biomediche e un rappresentante dei cittadini. L’obiettivo del meeting era rivedere e ampliare la checklist e il diagramma di flusso del QUOROM.
Durante la preparazione del meeting il comitato esecutivo ha condotto una revisione sistematica degli studi sulla qualità del reporting delle revisioni sistematiche, oltre a una esaustiva revisione della letteratura per identificare articoli, metodologici e non, potenzialmente utili per modificare gli item della checklist. Inoltre, è stata condotta anche una survey internazionale tra autori di revisioni, utenti e gruppi che commissionano o utilizzano revisioni sistematiche e meta-analisi, tra cui l’International Network of Agencies for Health Technology Assessment (INAHTA) e il Guidelines International Network (GIN). La survey mirava a raccogliere opinioni sul QUOROM, in particolare sulla validità degli item della checklist. I risultati di queste attività , presentati durante l’incontro, sono disponibili sul sito web del PRISMA (www.prisma-statement.org).
Anche se sono stati mantenuti o aggiunti alla checklist solo item ritenuti essenziali, ne esistono altri opzionali che gli autori di revisioni sistematiche dovrebbero includere, se rilevanti (10). Ad esempio, è utile indicare se la revisione sistematica rappresenta l’aggiornamento (11) di una precedente, descrivendo ogni modifica nei metodi, rispetto a quelli riportati nel protocollo originale.
Dopo il meeting è stata fatta circolare una bozza della checklist PRISMA sia tra i componenti del gruppo, sia tra le persone invitate che non avevano partecipato. è stato redatto un documento condiviso che riportava commenti e revisioni e la checklist è stata quindi sottoposta a 11 revisioni. Infine, il gruppo ha approvato la checklist, il diagramma di flusso e questo articolo.
In assenza di evidenze dirette per giustificare il mantenimento o l’aggiunta di alcuni item, sono state considerate rilevanti evidenze estrapolate da altri ambiti. Ad esempio, l’item 5 richiede agli autori di fornire le informazioni di registrazione della revisione sistematica, incluso il numero di registrazione se disponibile. Anche se la registrazione delle revisioni sistematiche non è ancora molto diffusa (12,13) le riviste che aderiscono all’International Committee of Medical Journal Editors (ICMJE) (14) oggi richiedono che tutti gli studi clinici siano registrati con l’obiettivo di incrementare trasparenza e responsabilità (15). La registrazione potrebbe offrire verosimilmente grandi vantaggi ai revisori, evitando la duplicazione di revisioni sistematiche che affrontano lo stesso quesito (16,17) e assicurando una maggior trasparenza nel processo di aggiornamento.
Il PRISMA STATEMENT
È costituito da una checklist di 27 item (tabella 1) che ha l’obiettivo di guidare gli autori nel migliorare il reporting di revisioni sistematiche e meta-analisi. Anche se il focus del PRISMA è rappresentato dalle revisioni sistematiche di trial randomizzati, lo strumento può essere utilizzato anche come traccia per il reporting di revisioni sistematiche di altri tipi di studi, in particolare quelli che valutano l’efficacia degli interventi sanitari. Anche se il PRISMA Statement può essere utile per l’analisi critica di revisioni sistematiche pubblicate, la checklist PRISMA non rappresenta formalmente uno strumento per valutare la qualitĂ delle revisioni sistematiche.
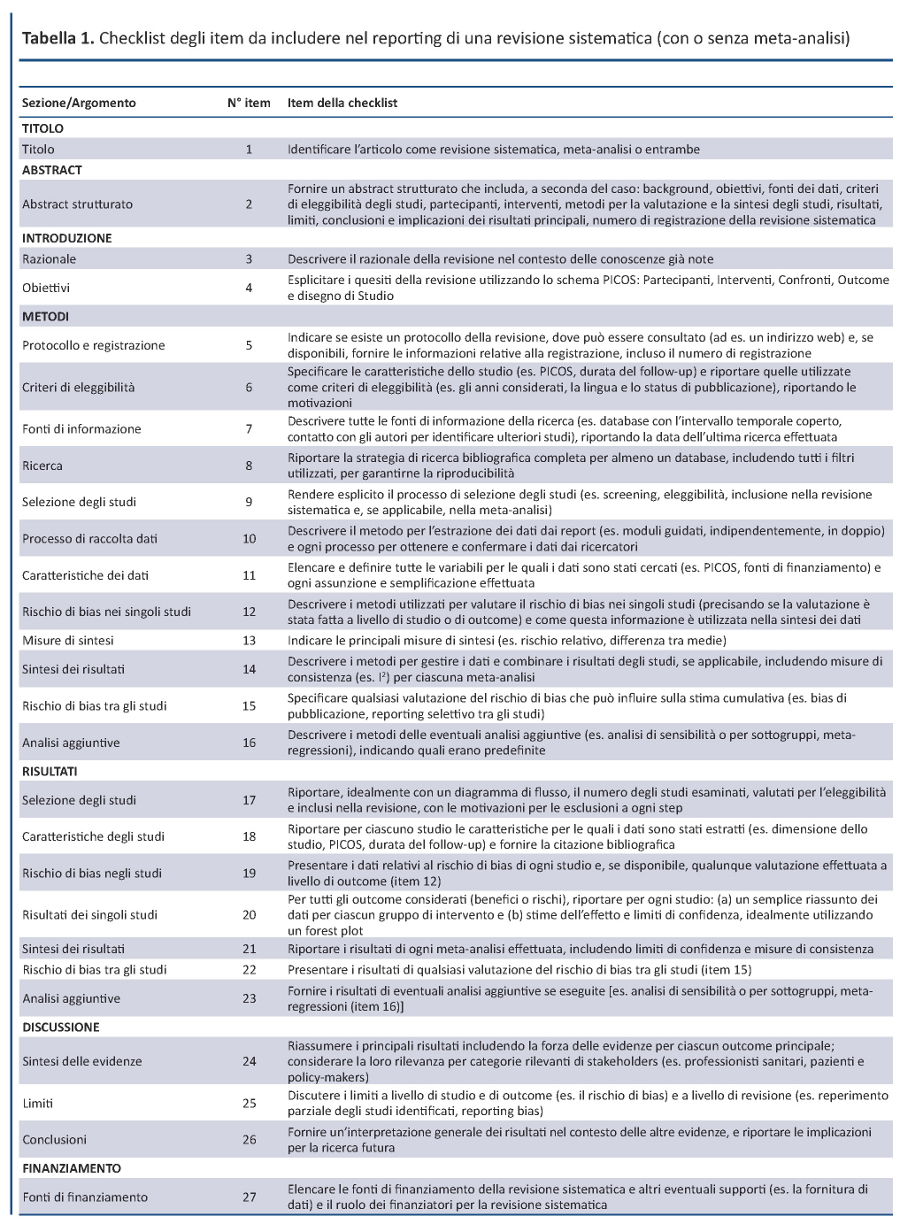
DAL QUOROM AL PRISMA
La checklist PRISMA presenta numerose differenze da quella QUOROM, di cui le principali sono riportate nella tabella 2. In generale, la checklist PRISMA scinde diversi item della checklist QUOROM e, dove possibile, ne accorpa altri per migliorare la coerenza del report di una revisione sistematica.
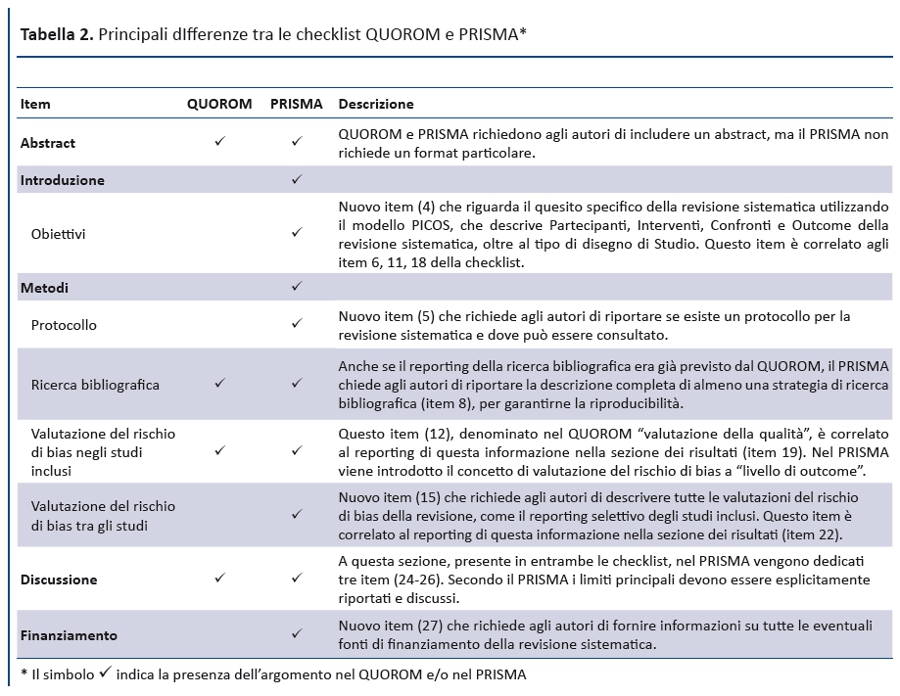
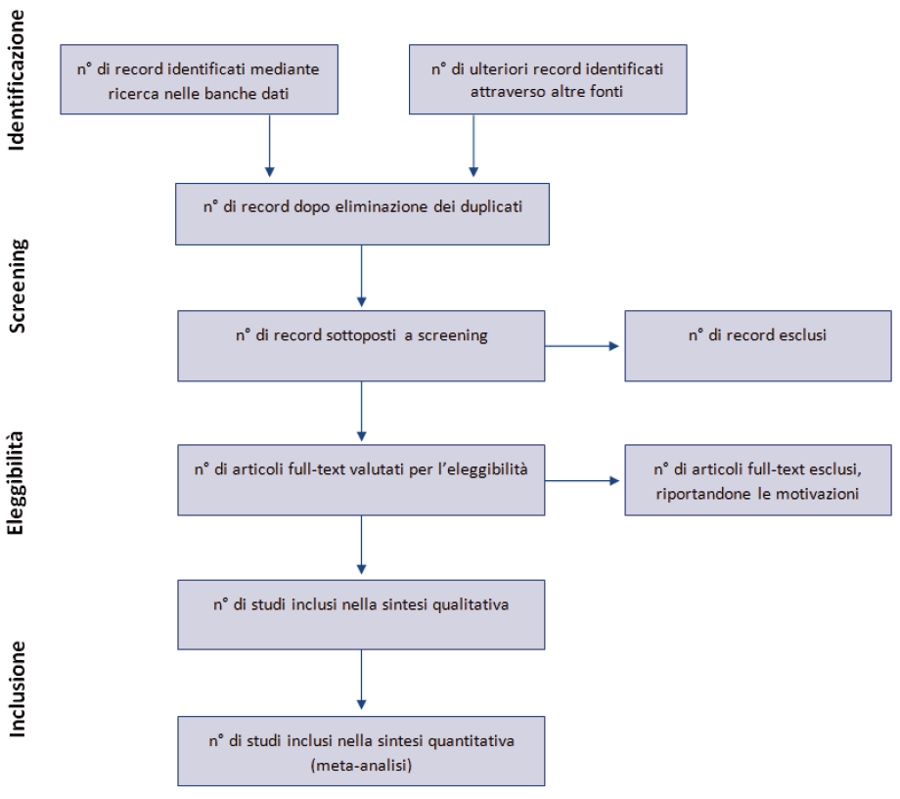
Anche il diagramma di flusso (figura 1) è stato modificato: prima di includere gli studi in una revisione sistematica e fornire le motivazioni per escluderne altri, gli autori devono effettuare una esaustiva ricerca bibliografica. Una volta selezionati i risultati e applicati i criteri di eleggibilità , rimarrà un numero esiguo di citazioni bibliografiche. Il numero degli articoli inclusi potrebbe essere minore (o maggiore) del numero degli studi, sia perché un articolo può riportare i risultati di diversi studi, sia perché i risultati di uno studio possono essere stati pubblicati in diversi articoli. Al fine di identificare correttamente queste informazioni, il diagramma di flusso del PRISMA richiede di documentare tutte le fasi del processo di revisione.
SUPPORTO
Il PRISMA Statement deve sostituire il QUOROM Statement per quelle riviste che lo hanno supportato. Ci auguriamo che altre riviste supportino il PRISMA, registrandosi sul sito. Per ribadire agli autori e ad altri soggetti l’importanza di un reporting trasparente delle revisioni sistematiche, incoraggiamo le riviste che supportano il PRISMA Statement a citarlo, riportando l’indirizzo web nelle istruzioni per gli autori. Invitiamo anche gli editori a supportare il PRISMA Statement e incoraggiamo gli autori ad aderire ai suoi principi.
ARTICOLO DI SPIEGAZIONE ED ELABORAZIONE
Analogamente ad altre linee guida per il reporting (18-20), il PRISMA Statement è accompagnato da un articolo di Spiegazione e Elaborazione (21). Per la sua stesura è stato sviluppato un ampio database di esempi di reporting ottimale per ciascun item della checklist e identificata una esaustiva evidence-base per supportare l’inclusione di ogni item della checklist. L’articolo di Spiegazione e Elaborazione è stato finalizzato attraverso diversi meeting e numerosi confronti a distanza tra i partecipanti del gruppo; il documento è stato quindi condiviso con il gruppo esteso per ulteriori revisioni e approvazione finale. Infine, il gruppo ha costituito un team specifico per la disseminazione e l’implementazione del PRISMA.
DISCUSSIONE
La qualità del reporting delle revisioni sistematiche non è ancora ottimale (22-27). Una recente valutazione di 300 revisioni sistematiche dimostra che solo raramente viene riportata la valutazione di un possibile bias di pubblicazione (22), nonostante l’incontrovertibile evidenza della sua esistenza (28) e del suo impatto sui risultati (29).
Anche se la possibilità del bias di pubblicazione viene valutata, non è certo che sia stata fatta e interpretata in maniera adeguata (30). Se la valutazione non è riportata non significa necessariamente che non è stata effettuata; tuttavia, se viene riportata, è più probabile che la revisione sia stata condotta in maniera accurata.
Sono state sviluppati numerosi approcci per condurre revisioni sistematiche su un’ampia varietà di quesiti: costo-efficacia (31), diagnosi (32), prognosi (33), associazioni genetiche (34) e policy making (35). I concetti generali e gli argomenti discussi nel PRISMA sono rilevanti per tutte le revisioni sistematiche, non solo per quelle che sintetizzano benefici e rischi di un intervento sanitario. Tuttavia, in particolari circostanze potrebbe essere necessaria qualche modifica agli item della checklist o al diagramma di flusso. Ad esempio, valutare il rischio di bias è un concetto generale, ma è verosimile che gli item utilizzati in una revisione sistematica di studi diagnostici si concentrino su altri aspetti (es. lo spettro dei pazienti, la verifica dello stato di malattia), diversamente da quelle che valutano l’efficacia di un intervento sanitario. Anche il diagramma di flusso richiede degli aggiustamenti quando si riportano meta-analisi di dati di pazienti individuali (36).
Per aumentare l’utilità del PRISMA abbiamo realizzato l’articolo di Spiegazione ed Elaborazione (18) che per ogni item della checklist contiene un esempio di reporting ottimale, il razionale per la sua inclusione e, ove disponibili, le evidenze a supporto con relativi riferimenti bibliografici. Questo articolo sarà utile anche a chi insegna la metodologia delle revisioni sistematiche. Invitiamo le riviste a includere il riferimento bibliografico al documento esplicativo nelle loro istruzioni per gli autori.
Considerato che, come ogni prodotto evidence-based, il PRISMA è un documento in continua evoluzione, invitiamo i lettori a commentare attraverso il sito web la versione rivista, in particolare la nuova checklist e il diagramma di flusso. Queste informazioni saranno utilizzate per lo sviluppo e l’aggiornamento continuo del PRISMA.
Strumenti
Figura S1. Diagramma di flusso relativo agli step di una revisione sistematica.
Testo S1. Checklist degli item da includere nel reporting di una revisione sistematica (con o senza meta-analisi)
Contributo degli Autori
Hanno letto e soddisfano i criteri ICMJE per l’authorship: DM, AL, JT, DGA. Hanno redatto la prima bozza dell’articolo: DM, AL, DGA. Hanno contribuito alla stesura dell’articolo: DM, AL, JT, DGA. Hanno partecipato alle periodiche conference call, hanno identificato i partecipanti, reperito i fondi, pianificato e partecipato ai meeting e redatto la bozza del manoscritto: DM, AL, DGA. Ha contribuito a identificare la evidence-base per il PRISMA, a perfezionare la checklist e a redigere la bozza del manoscritto: JT. Approvano le raccomandazioni: DM, AL, JT, DGA.
Disclosure dei conflitti di interesse
Nessuno dichiaratoIndirizzo per la corrispondenza
dmoher@ohri.caProvenienza
Tradotto con permesso da: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 2009;6(7): e1000097.Fonti di finanziamento
Canadian Institutes of Health Research; Università di Modena e Reggio Emilia (Italia), Cancer Research UK; Clinical Evidence BMJ Knowledge; The Cochrane Collaboration; GlaxoSmithKline. AL è parzialmente finanziato dal Ministero Italiano dell’Università e della Ricerca (COFIN-PRIN 2002 prot. 2002061749 e COFIN-PRIN 2006 prot. 2006062298). DGA è finanziato dal Cancer Research UK. DM è finanziato dall’Università di Ottawa. Gli sponsor non hanno avuto alcun coinvolgimento nella pianificazione, sviluppo o redazione dei documenti PRISMA, né alcun ruolo nella stesura del manoscritto.Approvazione comitato etico
-Ringraziamenti
Le seguenti persone hanno contribuito al PRISMA Statement: Doug Altman, DSc, Centre for Statistics in Medicine (Oxford, UK); Gerd Antes, PhD, University Hospital Freiburg (Friburgo, Germania); David Atkins, MD, MPH, Health Services Research and Development Service, Veterans Health Administration (Washington, D. C., USA); Virginia Barbour, MRCP, DPhil, PLoS Medicine (Cambridge, UK); Nick Barrowman, PhD, Children’s Hospital of Eastern Ontario (Ottawa, Canada); Jesse A. Berlin, ScD, Johnson & Johnson Pharmaceutical Research and Development (Titusville, New Jersey, USA); Jocalyn Clark, PhD, PLoS Medicine (al momento della stesura, BMJ, Londra, UK); Mike Clarke, PhD, UK Cochrane Centre (Oxford, UK) and School of Nursing and Midwifery, Trinity College (Dublino, Irlanda); Deborah Cook, MD, Departments of Medicine, Clinical Epidemiology and Biostatistics, McMaster University (Hamilton, Canada); Roberto D’Amico, PhD, Università di Modena e Reggio Emilia (Modena, Italia) and Centro Cochrane Italiano, Istituto Ricerche Farmacologiche Mario Negri (Milano, Italia); Jonathan J. Deeks, PhD, University of Birmingham (Birmingham, UK); P. J. Devereaux, MD, PhD, Departments of Medicine, Clinical Epidemiology and Biostatistics, McMaster University (Hamilton, Canada); Kay Dickersin, PhD, Johns Hopkins Bloomberg School of Public Health (Baltimore, Maryland, USA); Matthias Egger, MD, Department of Social and Preventive Medicine, University of Bern (Berna, Svizzera); Edzard Ernst, MD, PhD, FRCP, FRCP(Edin), Peninsula Medical School (Exeter, UK); Peter C. Gøtzsche, MD, MSc, The Nordic Cochrane Centre (Copenhagen, Danimarca); Jeremy Grimshaw, MBChB, PhD, FRCFP, Ottawa Hospital Research Institute (Ottawa, Canada); Gordon Guyatt, MD, Departments of Medicine, Clinical Epidemiology and Biostatistics, McMaster University (Hamilton, Canada); Julian Higgins, PhD, MRC Biostatistics Unit (Cambridge, UK); John P. A. Ioannidis, MD, University of Ioannina Campus (Ioannina, Grecia); Jos Kleijnen, MD, PhD, Kleijnen Systematic Reviews Ltd (York, UK) and School for Public Health and Primary Care (CAPHRI), University of Maastricht (Maastricht, Paesi Bassi); Tom Lang, MA, Tom Lang Communications and Training (Davis, California, USA); Alessandro Liberati, MD, Università di Modena e Reggio Emilia (Modena, Italia) and Centro Cochrane Italiano, Istituto Ricerche Farmacologiche Mario Negri (Milan, Italy); Nicola Magrini, MD, NHS Centre for the Evaluation of the Effectiveness of Health Care – CeVEAS (Modena, Italy); David McNamee, PhD, The Lancet (London, UK); Lorenzo Moja, MD, MSc, Centro Cochrane Italiano, Istituto Ricerche Farmacologiche Mario Negri (Milano, Italia); David Moher, PhD, Ottawa Methods Centre, Ottawa Hospital Research Institute (Ottawa, Canada); Cynthia Mulrow, MD, MSc, Annals of Internal Medicine (Philadelphia, Pennsylvania, USA); Maryann Napoli, Center for Medical Consumers (New York, New York, USA); Andy Oxman, MD, Norwegian Health Services Research Centre (Oslo, Norvegia); Ba’ Pham, MMath, Toronto Health Economics and Technology Assessment Collaborative (Toronto, Canada) (al momento del primo meeting del gruppo, GlaxoSmithKline Canada, Mississauga, Canada); Drummond Rennie, MD, FRCP, FACP, University of California San Francisco (San Francisco, California, USA); Margaret Sampson, MLIS, Children’s Hospital of Eastern Ontario (Ottawa, Canada); Kenneth F. Schulz, PhD, MBA, Family Health International (Durham, North Carolina, USA); Paul G. Shekelle, MD, PhD, Southern California Evidence Based Practice Center (Santa Monica, California, USA); Jennifer Tetzlaff, BSc, Ottawa Methods Centre, Ottawa Hospital Research Institute (Ottawa, Canada); David Tovey, FRCGP, The Cochrane Library, Cochrane Collaboration (Oxford, UK) (al momento del primo meeting del gruppo, BMJ, Londra, UK); Peter Tugwell, MD, MSc, FRCPC, Institute of Population Health, University of Ottawa (Ottawa, Canada).
Note alla versione italiana
La Fondazione GIMBE ha sostenuto la traduzione italiana dell’articolo senza alcun supporto istituzionale o commerciale.
Team che ha realizzato la versione italiana
Responsabile scientifico:
Antonino Cartabellotta, Fondazione GIMBE
Coordinamento editoriale:
Marco Mosti, Fondazione GIMBE
Traduzione:
Elena Cottafava, Fondazione GIMBE
Marco Da Roit, Fisioterapista, UO Medicina Riabilitativa dell'Azienda Ospedaliera-Universitaria di Ferrara
Revisione editoriale:
Roberto Luceri, Fondazione GIMBE
Manuela Sola, Fondazione GIMBE
Pagina aggiornata il 27/giugno/2015



