Guidelines & Standards
Evidence 2017;9(2): e1000161 doi: 10.4470/E1000161
Pubblicato: 23 febbraio 2017
Copyright: © 2017 Chen et al. Questo è un articolo open-access, distribuito con licenza Creative Commons Attribution, che ne consente l’utilizzo, la distribuzione e la riproduzione su qualsiasi supporto esclusivamente per fini non commerciali, a condizione di riportare sempre autore e citazione originale.
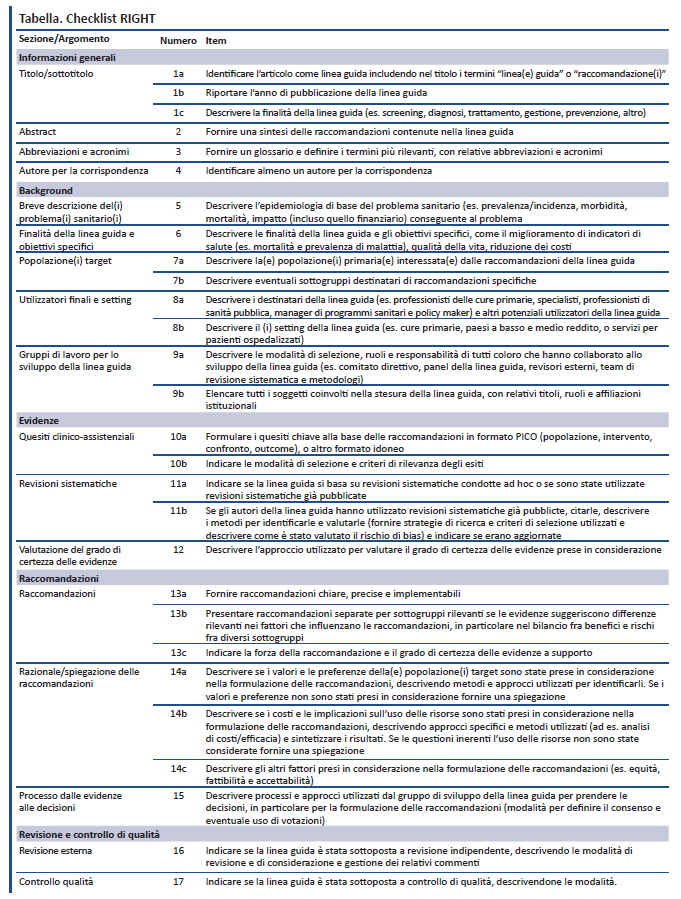
La qualità del reporting delle linee guida (LG) per la pratica clinica è spesso insoddisfacente e non esistono indicazioni o standard universalmente accettati. Il gruppo di lavoro internazionale RIGHT (Reporting Items for practice Guidelines in HealThcare) – costituito per colmare questo gap – si è ispirato a un modello esistente per lo sviluppo di LG per il reporting della ricerca biomedica e all’approccio del network EQUATOR (Enhancing the QUAlity and Transparency Of health Research). Il gruppo ha sviluppato una checklist e un documento di spiegazione ed elaborazione. La checklist contiene 22 item considerati essenziali per un buon reporting delle LG: informazioni generali (item 1-4), background (item 5-9), evidenze (item 10-12), raccomandazioni (item 13-15), revisione e controllo di qualità (item 16-17), fonti di finanziamento, disclosure e gestione dei conflitti d’interesse (item 18-19), altre informazioni (item 20-22). La checklist RIGHT può aiutare gli autori di LG nel reporting, supportare gli editori e i revisori delle riviste biomediche nel valutare i report di LG e i professionisti sanitari nel comprendere e implementare le LG nella pratica clinica.
Linee guida (LG) chiare, esplicite e trasparenti permettono a professionisti sanitari, decisori e cittadini di comprendere e implementare nella pratica le raccomandazioni cliniche che possono influenzare positivamente la salute delle persone e di popolazioni differenti (1). Tuttavia la qualità con cui vengono riportate le LG sembra essere inadeguata (2) e gli strumenti attualmente utilizzati per gestire questo problema sono obsoleti, o troppo circoscritti, o, ancora, combinano elementi di valutazione della qualità delle LG con altri di valutazione del reporting. La Conference on Guideline Standardization ha pubblicato una checklist per il reporting delle LG (ultimo aggiornamento nel 2003) mirata principalmente alla medicina clinica e che pertanto può non essere direttamente applicabile a LG di sanità pubblica o ad altre LG (3). Lo strumento AGREE (Appraisal of Guidelines for REsearch and Evaluation) è stato sviluppato per valutare sia la qualità sia il reporting delle LG, sebbene sia generalmente considerato uno strumento per valutarne la qualità (4,5). Strumenti multifunzionali possono non essere ottimali e devono essere mantenuti distinti dalle checklist che valutano la qualità del reporting delle LG e da quelle che ne valutano la qualità metodologica in quanto differenti per obiettivi, struttura e contenuto (6). Recentemente l’AGREE Next Steps Consortium ha pubblicato la checklist AGREE per il reporting delle LG basata sullo strumento AGREE (7,8). Questa checklist, tuttavia – limitata agli item derivati dallo strumento originario – è stata sviluppata da un gruppo ristretto di ricercatori e non fornisce spiegazioni dettagliate, nè una guida all’utilizzo.
Sviluppo della checklist RIGHT
Nel 2013 si è costituito un gruppo internazionale multidisciplinare di policy maker, metodologi, epidemiologi, clinici, editori e rappresentanti dei cittadini rappresentativi di 12 paesi di Asia, Africa, Europa, Oceania e Nord America. Lo scopo era sviluppare la checklist RIGHT (Reporting Items for practice Guidelines in HealThcare), un elenco di item essenziali per il reporting delle LG. Lo sviluppo della checklist si è basato sul metodo utilizzato per le LG sul reporting della ricerca biomedica (9) e il progetto è stato registrato nella EQUATOR Library (Enhancing the QUAlity and Transparency Of health Research) (10). Il gruppo di lavoro RIGHT ha prodotto la bozza del progetto, redatto la lista di item suggeriti, reclutato i componenti del gruppo Delphi, prodotto i questionari per la survey Delphi e stilato la bozza del manoscritto finale. Il gruppo Delphi ha revisionato la proposta, partecipato a tre round di consultazioni Delphi, raggiunto un consenso sulle voci incluse nella checklist definitiva e ha revisionato il manoscritto finale.
Il gruppo di lavoro RIGHT ha utilizzato un approccio a 4 step per generare gli item potenziali della checklist. Innanzitutto il gruppo ha esaminato 10 rilevanti LG per il reporting tra le risorse di EQUATOR per determinare come erano stati generati i potenziali item (11). Queste LG per il reporting erano riferite a varie tipologie di studi: trial controllati randomizzati, studi di accuratezza diagnostica, studi osservazionali, ricerca su animali, valutazioni economiche, revisioni sistematiche. Uno di questi strumenti aveva generato gli item basandosi su una revisione sistematica (12), mentre altri si erano basati su survey, meeting di gruppi, revisioni di letteratura o approcci combinati (13-21). In secondo luogo è stata condotta una ricerca estesa di manuali e altri documenti per identificare standard o strumenti per il reporting delle LG (Appendice 3, Figura in Appendice e Tabella in Appendice). Quindi 2 sottogruppi, ciascuno con 2 ricercatori esperti, hanno estratto in maniera indipendente i potenziali item per la checklist da tutti i documenti identificati nelle due fasi precedenti. Infine, l’intero gruppo si è riunito per aggregare tutti gli item potenziali e rimuovere i duplicati: 48 potenziali item sono stati inclusi nell’elenco iniziale. I lettori possono effettuare il download dei risultati della ricerca e la lista iniziale degli item dal sito web RIGHT (22).
Per la survey Delphi sono stati identificati 17 esperti di sviluppo o reporting delle LG, rappresentativi di varie discipline e provenienze geografiche. Il metodo Delphi, secondo le raccomandazioni di Murpy et coll. (23) e Sinha et coll. (24), ha incluso tre round di consultazioni via e-mail. I membri del gruppo hanno assegnato a ciascun item un punteggio da 1 (irrilevante) a 5 (molto rilevante), hanno suggerito nuovi item e fornito commenti condivisi nei successivi round di consultazione. Prima della survey Delphi i partecipanti hanno dichiarato eventuali conflitti d’interesse. Le percentuali di risposta sono state del 100% per tutti i round di consultazione.
Questo studio è stato finanziato dalla National Natural Science Foundation of China, che non ha avuto alcun ruolo nel disegno, nell’analisi e raccolta dei dati, nella stesura del manoscritto o nella decisione di pubblicarlo.
Descrizione della checklist
La checklist RIGHT include 22 item considerati essenziali per un adeguato reporting delle LG (Tabella). Gli item abbracciano i seguenti domini: informazioni generali (item 1-4), background (item 5-9), evidenze (item 10-12), raccomandazioni (item 13-15), revisione e controllo di qualità (item 16-17), fonti di finanziamento, disclosure e gestione dei conflitti d’interesse (item 18-19), altre informazioni (item 20-22). Per ciascun item della checklist il documento RIGHT di spiegazione e elaborazione (disponibile a: www.annals.org/data/Journals/AIM/935994/M16-1565_Supplement.pdf) fornisce ai lettori il razionale, ampie note esplicative ed esempi di reporting adeguato.
Discussione
La checklist RIGHT può aiutare gli autori nel reporting delle LG, supportare gli editori delle riviste biomediche e i revisori nel valutarle e aiutare i professionisti sanitari ad interpretarle e implementarle. La checklist è applicabile a tutte le LG, incluse quelle di sanità pubblica o altri ambiti sanitari. Essa fornisce ad utilizzatori e valutatori delle LG una descrizione chiara ed esplicita di processi e procedure utilizzate per lo sviluppo delle LG e dà accesso alle evidenze utilizzate per formulare le raccomandazioni.
La checklist RIGHT non prescrive uno specifico format per presentare le LG, ma piuttosto ogni item dovrebbe essere chiaramente presentato e sufficientemente dettagliato in qualche punto della LG. Ordine e formato degli item dipendono dalle preferenze dell’autore, dallo stile di pubblicazione e, in particolare, dalle necessità dei destinatari finali. La checklist non deve essere utilizzata per assegnare uno score di qualità: gli item non hanno la medesima rilevanza e gli score risultano inaffidabili quando utilizzati per gli studi di ricerca secondaria (25,26).
La checklist RIGHT non è stata sviluppata per valutare la qualità delle LG: esistono già strumenti con questa finalità, come AGREE II (27) e altri (28), ai quali la checklist RIGHT intende essere complementare. Inoltre, la checklist RIGHT non è uno strumento di supporto allo sviluppo delle LG, per cui esistono molti manuali, oltre alla checklist del Guidelines International Network-McMaster, uno strumento pratico per sviluppare LG supportato da risorse di apprendimento (29). I lettori di fatto dovrebbero selezionare attentamente uno strumento in relazione a loro specifiche necessità.
La checklist RIGHT differisce in maniera rilevante dall’AGREE reporting checklist (8). In primo luogo, la struttura di quest’ultima segue i domini di AGREE II relativi ad ambito e obiettivi, coinvolgimento degli stakeholder, rigore di sviluppo, chiarezza di presentazione, applicabilità e indipendenza editoriale. Viceversa, la checklist RIGHT segue l’approccio utilizzato in altri reporting statement, come il CONSORT (Consolidated Standards of Reporting Trials) (15) e il PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) (13) ed elenca gli item nell’ordine in cui vengono solitamente riportati in una LG. In secondo luogo, la checklist RIGHT include item rilevanti non previsti dall’AGREE reporting checklist: controllo di qualità, accessibilità, suggerimenti per la ricerca futura, limiti della LG. La checklist RIGHT sottolinea l’importanza di riportare i quesiti PICO (popolazione, intervento, confronto ed esiti) e la qualità delle evidenze e include 7 sotto-item riguardanti la formulazione delle raccomandazioni a partire dalle evidenze disponibili. Infine, il documento RIGHT di spiegazione ed elaborazione fornisce informazioni ed esempi dettagliati non presenti nell’AGREE reporting checklist.
L’adozione e l’implementazione di LG per il reporting può contribuire a ridurre gli sprechi nella ricerca e aumentare i potenziali effetti positivi sulla salute (30). Intendiamo promuovere in vari modi la checklist RIGHT, ad esempio invitando gli autori di manuali internazionali sulla produzione di LG ad aggiungere la checklist RIGHT alle versioni aggiornate dei loro manuali, contattando gli editori delle principali riviste biomediche indicizzate su MEDLINE (www.nlm.nih.gov/bsd/aim.html) per sollecitare il loro supporto ed incoraggiarli ad adottare la checklist, informando i produttori di LG presso agenzie nazionali e società scientifiche del progetto RIGHT.
Per produrre la checklist RIGHT abbiamo seguito un processo esplicito, trasparente e documentato, oltre che predisposto il documento di spiegazione ed elaborazione. A questo progetto hanno contribuito ricercatori di prestigiose organizzazioni e istituzioni internazionali dedicate allo sviluppo e implementazione delle LG, quali l’EQUATOR Network, il Guidelines International Network; il gruppo di lavoro GRADE (Grading of Recommendations Assessment, Development and Evaluation); l’AGREE Collaboration e la Cochrane Collaboration. La bozza della checklist e il documento di spiegazione ed elaborazione sono stati sottoposti ad una estesa peer review esterna da parte di esperti nello sviluppo di LG con differenti prospettive. Se nella prima versione della checklist possiamo aver tralasciato item rilevanti, abbiamo poi lavorato per minimizzare questo limite esaminando molti documenti e manuali pubblicati da produttori di LG e consultando un ampio gruppo di esperti del settore.
La checklist RIGHT è disponibile in lingua inglese, tedesca, croata, giapponese, coreana e cinese tradizionale e semplificato e ulteriori traduzioni sono benvenute. Stiamo pianificando lo sviluppo di alcune estensioni del RIGHT, compreso il RIGHT-P (per le proposte di LG), RIGHT-COI (per il conflitto di interessi), e il RIGHT-A (per l’agopuntura). Chiediamo a tutti coloro che intendono produrre standard correlati o traduzioni di contattare gli autori di questo articolo per coordinare gli sforzi ed evitare duplicazioni.
Come ogni altra LG per il reporting, la checklist RIGHT è un documento vivo che necessita di continue valutazioni, miglioramenti e aggiornamenti. Rivedremo in futuro la checklist basandoci su feedback degli utilizzatori, risultati di valutazioni formali e informali, nuovi studi sulle metodologie di reporting delle LG. Incoraggiamo gli utilizzatori a inviare i loro feedback attraverso il sito web RIGHT.
Disclaimer
Le opinioni e le conclusioni espresse dagli autori in questo articolo non rappresentano necessariamente la posizione dell’Organizzazione Mondiale della Sanità e dei Centers for Disease Control and Prevention.
Appendice 1. Membri del gruppo di lavoro RIGHT
Membri del gruppo di lavoro RIGHT che hanno prodotto questo documento: Yaolong Chen, PhD, MMed, Kehu Yang, MMed, Jinhui Tian, PhD, and Qi Wang, MMed (Lanzhou University, Lanzhou, Gansu, Cina); Susan L. Norris, MD, MPH, MSc (World Health Organization, Ginevra, Svizzera); Ana Marušic , MD, PhD (University of Split, Spalato, Croazia); Amir Qaseem, MD, PhD, MHA (American College of Physicians, Philadelphia, Pennsylvania); Joerg J. Meerpohl, MD (Paris–Sorbonne University, Parigi, Francia); Signe Flottorp, MD, PhD (Norwegian Institute of Public Health, Oslo, Norvegia); Elie A. Akl, MD, MPH, PhD (American University of Beirut, Beirut, Libano); Holger J. Schünemann, MD, PhD (McMaster University, Hamilton, Ontario, Canada); Edwin S.Y. Chan, PhD (Cochrane Singapore, Biopolis, Singapore); Yngve Falck-Ytter, MD (Case Western Reserve University); Faruque Ahmed, PhD (Centers for Disease Control and Prevention, Atlanta, Georgia); Sarah Barber, PhD (World Health Organization Regional Office for Africa, Brazzaville, Repubblica del Congo); Chiehfeng Chen, MD, MPH, PhD (Taipei Medical University – Municipal Wan Fang Hospital, Taipei, Taiwan); Mingming Zhang, MSc (Chinese Cochrane Centre, Sichuan, Cina); Bin Xu, MD (Nanjing University of Chinese Medicine, Nanjing, Cina); Fujian Song, PhD (University of East Anglia, Norwich, Regno Unito); Hongcai Shang, MD, PhD (Dongzhimen Hospital of Beijing University of Chinese Medicine, Pechino, Cina); e Kun Tang, PhD (Peking University, Pechino, Cina).
Membri del gruppo RIGHT che non hanno collaborato alla stesura di questo documento: Hui Li, MD, PhD (Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, Guangdong, Cina); Yan Hu, MD, PhD, e Boheng Zhang, MD, PhD (Fudan University, Shanghai, Cina); Huan Shen, MD, PhD, e Li Jiang, MD, PhD (Peking University People’s Hospital, Pechino, Cina); Suodi Zhai, MD, PhD (Peking University Third Hospital, Pechino, Cina); e Xufei Luo, MBBS, e Yanfang Ma, MBBS (Lanzhou University, Lanzhou, Gansu, Cina).
Appendice 2: Contributo degli autori
I Dr. Y. Chen e Norris e il Prof. Yang hanno ideato il progetto RIGHT e stilato la bozza del progetto. I Dr. Marušic, Qaseem, Meerpohl, Flottorp, Akl, Chan, Falck-Ytter, Ahmed, Barber, C. Chen, and Xu and Ms. Zhang sono stati componenti del gruppo Delphi e hanno fornito suggerimenti e commenti alla bozza di lista degli item della checklist. I Dr. Y. Chen, Song e Tang, il Prof. Yang e Ms. Wang hanno generato gli item proposti, formulato i questionari per la survey Delphi ed effettuato l’analisi statistica. I Dr. Y. Chen e Norris hanno stilato il manoscritto. Tutti gli autori hanno effettuato una revisione critica e verifica del manoscritto nei suoi rilevanti contenuti intellettuali. Il Dr. Y. Chen, garante del documento, afferma che è un onesto, accurato e trasparente resoconto dello studio che viene riportato. Tutti gli autori hanno approvato la versione finale di questo articolo.
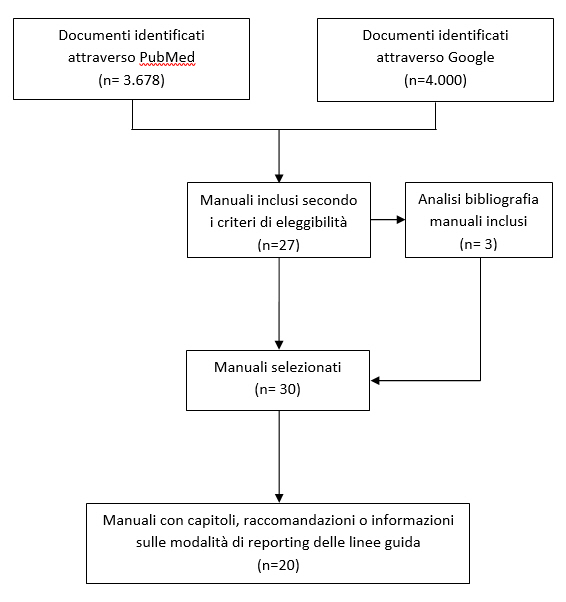
Appendice 3: Metodi e risultati della ricerca sistematica di manuali per la produzione di linee guida
Strategie di ricerca
Abbiamo condotto una ricerca sistematica in MEDLINE (attraverso PubMed, dal 1966 in poi) usando la seguente combinazione di termini:
#1 Clinical Practice Guideline*[tw]
#2 Clinical guideline*[tiab]
#3 Guideline*[ti]
#4 guidance*[ti]
#5 consensus[ti]
#6 recommendation*[ti]
#7 OR#1-#6
#8 methodolog*[tiab]
#9 handbook*[tiab]
#10 manual*[tiab]
#11 toolkit*[tiab]
#12 OR#8-#11
#13 #7 AND #12
#14 Practice Guideline[pt]
#15 #13 NOT #14
Il 30 aprile 2014 abbiamo effettuato anche una ricerca usando il motore di ricerca Google (Alphabet). Abbiamo aggiunto singolarmente i seguenti termini di ricerca in Google ed esaminati i primi 200 record per ciascuna stringa di ricerca:
#1 guideline methodolog*
#2 guideline handbook
#3 guideline manual
#4 guideline toolkit
#5 guidance methodolog*
#6 guidance handbook
#7 guidance manual
#8 guidance toolkit
#9 consensus methodolog*
#10 consensus handbook
#11 consensus manual
#12 consensus toolkit
#13 recommendation methodolog*
#14 recommendation handbook
#15 recommendation manual
#16 recommendation toolkit
#17 standard methodolog*
#18 standard handbook
#19 standard manual
#20 standard toolkit
Abbiamo controllato accuratamente le bibliografie di tutti i manuali inclusi e il materiale aggiuntivo non reperito tramite le strategie di ricerca sopra riportate. Inoltre abbiamo esaminato le bibliografie citate negli articoli di Ansari e Rashidin (31) e VernoiJ et coll. (32) identificati nella revisione della letteratura.
Criteri di eleggibilità
Abbiamo incluso i manuali che fornivano istruzioni sull’intero processo di sviluppo delle LG. Sono stati esclusi tutti i documenti scritti da singoli autori, le versioni precedenti di manuali successivamente aggiornati, i documenti focalizzati su aspetti specifici dello sviluppo delle LG (es. aggiornamento delle stesse, revisioni sistematiche, metodo GRADE). Sono stati inclusi manuali (solo in lingua inglese) che contenevano una sezione su come presentare, scrivere o effettuare il reporting di una LG.
Selezione dei manuali
Due revisori hanno valutato indipendentemente tutti i documenti identificati (Q.W. e K.T); i disaccordi sono stati risolti tramite consenso e, quando necessario, con il supporto di un team leader (Y.C.). Complessivamente sono stati inclusi 30 manuali di sviluppo delle LG, di cui 20 contenevano sezioni sulle modalità di reporting.

Contributo degli Autori
-Disclosure dei conflitti di interesse
Il dott. Meerpohl è membro del gruppo di lavoro GRADE e del comitato direttivo GRADE. La dott.ssa Flottorp è membro del gruppo di lavoro GRADE e del comitato direttivo GRADE. Il dott. Akl è membro del gruppo di lavoro GRADE. Il dott. Schünemann è il co-chair del gruppo di lavoro GRADE e autore principale della checklist di sviluppo delle linee guida elaborata dal Guidelines International Network e dalla McMaster. Il dott. Chan dichiara di percepire un compenso part time dal Ministero della Salute di Singapore per attività che esulano dal presente lavoro. Il dott. Falck-Ytter è membro del gruppo di lavoro GRADE. Gli altri autori non hanno dichiarato conflitti di interesse. Le disclosure sono disponibili a: www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M16-1565.
Indirizzo per la corrispondenza
yangkehuebm2006@163.com (Kehu Yang); scn5@cdc.gov (Susan L. Norris)Provenienza
Tradotto con permesso da Chen Y, Yang K, Marušic A, et al. RIGHT (Reporting Items for Practice Guidelines in Healthcare) Working Group. A Reporting Tool for Practice Guidelines in Health Care: The RIGHT Statement. Ann Intern Med 2017;166:128-132. Permission reference: PERM-121616-GIMBE-AM. L’American College of Physicians, che ha autorizzato la traduzione dell’articolo a fini non commerciali, non si assume alcuna responsabilità per l’accuratezza della traduzione.Fonti di finanziamento
National Natural Science Foundation of China (Dott. Chen, grant 81503459), China Fundamental Research Funds for the Central Universities (Dott. Chen e Prof. Yang, grant 2016LZUJBZX159), Open Fund of the Key Laboratory of Evidence-Based Medicine and Knowledge Translation of Gansu Province (grant EBM1305 per il progetto RIGHT), e Croatian Science Foundation (Dott. Marušic, grant IP-2014-09-7672).Approvazione comitato etico
-Ringraziamenti
Gli autori ringraziano tutti coloro che hanno partecipato alla survey Delphi per i loro rilevanti commenti.
Note alla versione italiana
La Fondazione GIMBE ha realizzato la traduzione italiana del RIGHT Statement senza alcun finanziamento istituzionale o commerciale.
Team che ha realizzato la versione italiana
Responsabile scientifico
Antonino Cartabellotta, Fondazione GIMBE
Coordinamento editoriale
Marco Mosti, Fondazione GIMBE
Traduzione
Primiano Iannone, Istituto Superiore di Sanità
Revisione editoriale
Elena Cottafava, Fondazione GIMBE
Pagina aggiornata il 23/febbraio/2017



