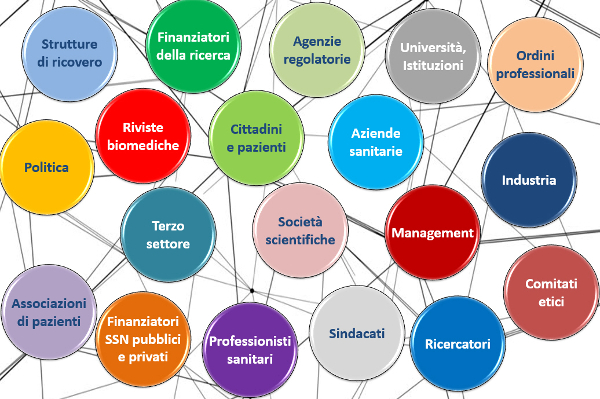
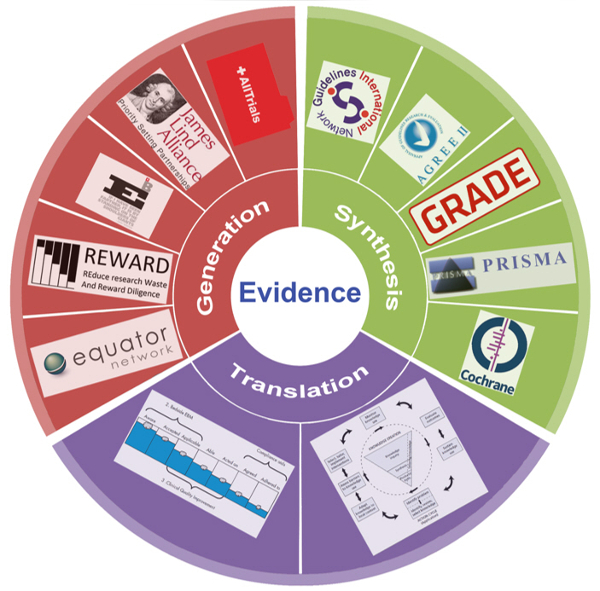
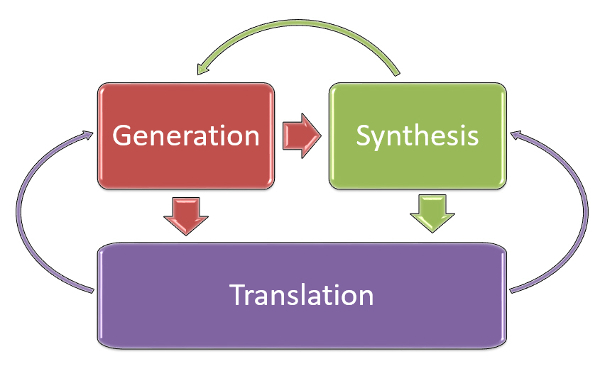
Conference Report
L’ecosistema delle evidenze scientifiche: produzione, sintesi e implementazione
Antonino CartabellottaEvidence 2017;9(9): e1000171 doi: 10.4470/E1000171
Pubblicato: 12 novembre 2017
Copyright: © 2017 Cartabellotta. Questo è un articolo open-access, distribuito con licenza Creative Commons Attribution, che ne consente l’utilizzo, la distribuzione e la riproduzione su qualsiasi supporto esclusivamente per fini non commerciali, a condizione di riportare sempre autore e citazione originale.
1. Evidence-Based Medicine Working Group. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA 1992;268:2420-5.
2. Djulbegovic B, Guyatt GH. Progress in evidence-based medicine: a quarter century on. Lancet 2017;390:415-423.
3. Ioannidis JPA. Hijacked evidence-based medicine: stay the course and throw the pirates overboard. J Clin Epidemiol 2017;84:11-13.
4. Greenhalgh T, Howick J, Maskrey N; Evidence Based Medicine Renaissance Group. Evidence based medicine: a movement in crisis? BMJ 2014;348:g3725.
5. Heneghan C, Mahtani KR, Goldacre B, Godlee F, Macdonald H, Jarvies D. Evidence based medicine manifesto for better healthcare. BMJ. 2017 Jun 20;357:j2973.
6. 8th International Conference for EBHC Teachers and Developers. The ecosystem of evidence: connecting generation, synthesys and translation. Disponibile a: www.ebhc.org. Ultimo accesso: 13 novembre 2017
7. Cartabellotta A. Ridurre gli sprechi e premiare il rigore scientifico nella ricerca biomedica: la campagna Lancet-REWARD. Evidence 2016;8(5): e1000142.
8. James Lind Alliance (JLA) Priority Setting Partnership (PSP). Disponibile a: www.jla.nihr.ac.uk/priority-setting-partnerships. Ultimo accesso: 13 novembre 2017
9. Lund H, Brunnhuber K, Juhl C, Robinson K, Leenaars M, Dorch BF, et al. Evidence-Based Research: le revisioni sistematiche devono sempre informare i nuovi studi primari. Evidence 2017;9(3): e1000164.
10. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jeric K, et al. SPIRIT Statement 2013: checklist per il protocollo dei trial clinici. Evidence 2016;8(8): e1000148 (30 agosto 2016).
11. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647.
12. Academy of Medical Sciences. Reproducibility and reliability of biomedical research: improving research practice. Symposium report, October 2015 Disponibile a: www.acmedsci.ac.uk/file-download/38190-56314fa158e14.pdf. Ultimo accesso: 13 novembre 2017
13. Chalmers I, Glasziou G, Godlee F. Tutti i trial devono essere registrati e tutti i risultati pubblicati. Evidence 2013;5(1): e1000032.
14. Organizzazione Mondiale della Sanità Rendere pubblici i risultati dei trial clinici: lo statement dell’Organizzazione Mondiale della Sanità . Evidence 2016;8(2): e1000134.
15. Laine C, Horton R, DeAngelis CD, Drazen JM, Frizelle FA, Godlee F, Haug C, HĂ©bert PC, Kotzin S, Marusic A, Sahni P, Schroeder TV, Sox HC, Van der Weyden MB, Verheugt FW. Clinical trial registration--looking back and moving ahead. Disponibile a: www.icmje.org/news-and-editorials/clincial_trial_reg_jun2007.html. Ultimo accesso: 13 novembre 2017
16. World Health Organization. International Clinical Trials Registry Platform (ICTRP). Disponibile a: www.who.int/ictrp. Ultimo accesso: 13 novembre 2017
17. Taichman DB, Sahni P, Pinborg A, Peiperl L, Laine C, James A, Hong ST, Haileamlak A, Gollogly L, Godlee F, Frizelle FA, Florenzano F, Drazen JM, Bauchner H, Baethge C, Backus J. Data Sharing Statements for Clinical Trials: A Requirement of the International Committee of Medical Journal Editors. Disponibile a: www.icmje.org/news-and-editorials/data_sharing_june_2017.pdf. Ultimo accesso 13 novembre 2017
18. EQUATOR Network. Enhancing the QUAlity and Transparency Of health Research. Disponibile a: www.equator-network.org. Ultimo accesso: 13 novembre 2017
19. Nasser M, Clarke M, Chalmers I, Brurberg KG, Nykvist H, Lund H, Glasziou P. What are funders doing to minimise waste in research? Lancet 2017;389:1006-1007.
20. Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res 2014;14:89.
21. Al-Shahi Salman R, Beller E, Kagan J, Hemminki E, Phillips RS, Savulescu J, Macleod M, Wisely J, Chalmers I. Increasing value and reducing waste in biomedical research regulation and management. Lancet 2014;383:176-85.
22. Ioannidis JP, Greenland S, Hlatky MA, Khoury MJ, Macleod MR, Moher D, Schulz KF, Tibshirani R. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014;383:166-75.
23. Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, GĂĽlmezoglu AM, Howells DW, Ioannidis JP, Oliver S. How to increase value and reduce waste when research priorities are set. Lancet 2014;383:156-65.
24. Evidence-Based Medicine Data Lab, University of Oxford. TrialsTracker. Disponibile a: https://trialstracker.ebmdatalab.net. Ultimo accesso: 13 novembre 2017
25. Goldacre B, Drysdale H, Powell-Smith A, et al. The COMPare Trials Project. Disponibile a: www.COMPare-trials.org. Ultimo accesso: 13 novembre 2017
26. Mannocci A, Saulle R, Colamesta V, D’Aguanno S, Giraldi G, Maffongelli E, Meggiolaro A, Semyonov L, Unim B, La Torre G. What is the impact of reporting guidelines on Public Health journals in Europe? The case of STROBE, CONSORT and PRISMA. J Public Health (Oxf) 2015;37:737-40.
27. Cochrane Training. Guides and handbooks. Disponibile a: www.training.cochrane.org/handbooks. Ultimo accesso. 13 novembre 2017
28. Cochrane Methods GRADEing group. Disponibile a: www.methods.cochrane.org/gradeing/welcome. Ultimo accesso: 13 novembre 2017
29. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Linee guida per il reporting di revisioni sistematiche e meta-analisi: il PRISMA Statement. Evidence 2015;7(6): e1000114
30. PRISMA Statement extensions. Disponibile a: www.prisma-statement.org/Extensions. Ultimo accesso: 13 novembre 2017
31. Ioannidis JP. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q 2016;94:485-514.
32. Cochrane Database of Systematic Reviews. Number of Cochrane reviews and protocols by issue. Disponibile a: www.cochranelibrary.com/dotAsset/5ed035c1-ae96-4139-be3a-7ca0065269a2.pdf. Ultimo accesso: 13 novembre 2017
33. Cochrane Database of Systematic Reviews. Impact factor. Disponibile a: www.cochranelibrary.com/cochrane-database-of-systematic-reviews. Ultimo accesso: 13 novembre 2017
34. CRD Databases. Changes to DARE and NHS EED. 11 May 2015. Disponibile a: www.crd.york.ac.uk/crdweb/newspage.asp. Ultimo accesso: 13 novembre 2017
35. Guidelines International Network (G-I-N). Disponibile a: www.g-i-n.net. Ultimo accesso: 13 novembre 2017
36. Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P; Board of Trustees of the Guidelines International Network. Guidelines International Network: verso standard internazionali per la produzione di linee guida. Evidence 2012;4(6): e1000022
37. Brouwers M, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna S, Littlejohns P, Makarski J, Zitzelsberger L for the AGREE Next Steps Consortium. AGREE II: Advancing guideline development, reporting and evaluation in healthcare. CMAJ 2010;182:E839-42.
38. Institute of Medicine. Clinical Practice Guidelines We Can Trust. Standards for Developing Trustworthy Clinical Practice Guidelines (CPGs). Washington (DC): National Academies Press (US); 2011.
39. Schünemann HJ, Al-Ansary LA, Forland F, Kersten S, Komulainen J, Kopp IB, Macbeth F, Phillips SM, Robbins C, van der Wees P, Qaseem A; Board of Trustees of the Guidelines International Network. Disclosure e gestione dei conflitti di interesse nelle linee guida: i princìpi del Guidelines International Network. Evidence 2016;8(3): e1000136.
40. GRADE Working Group. Disponibile a: www.gradeworkinggroup.org. Ultimo accesso: 13 novembre 2017
41. Cartabellotta A, LaganĂ AS. AGREE Reporting Checklist: uno strumento per migliorare il reporting delle linee guida. Evidence 2016;8(7): e1000146.
42. Chen Y, Yang K, Marušic A, Qaseem A, Meerpohl JJ, Flottorp S, Akl EA, Schünemann HJ, Chan ES, Falck-Ytter Y, Ahmed F, Barber S, Chen C, Zhang M, Xu B, Tian J, Song F, Shang H, Tang K, Wang Q, Norris SL; RIGHT (Reporting Items for Practice Guidelines in Healthcare) Working Group. RIGHT statement: strumento per il reporting delle linee guida per la pratica clinica. Evidence 2017;9(2): e1000161.
43. Vernooij RW, Alonso-Coello P, Brouwers M, MartĂnez GarcĂa L; CheckUp Panel. Reporting Items for Updated Clinical Guidelines: Checklist for the Reporting of Updated Guidelines (CheckUp). PLoS Med 2017;14:e1002207.
44. Cartabellotta A, Tedesco T, Pomponio G. Linee guida per la valutazione clinica e il trattamento dei pazienti con multimorbiditĂ . Evidence 2016;8(10): e1000154.
45. Glasziou P, Haynes B. The paths from research to improved health outcomes. ACP J Club 2005;142(2):A8-10.
46. Graham I, Straus S, Tetroe J. Knowledge Translation in Health Care: Moving from Evidence to Practice. John Wiley & Sons, 2013.
47. Ioannidis JP, Khoury MJ. Assessing value in biomedical research: the PQRST of appraisal and reward. JAMA 2014;312:483-4.
48. Cruz Rivera S, Kyte DG, Aiyegbusi OL, Keeley TJ, Calvert MJ. Assessing the impact of healthcare research: A systematic review of methodological frameworks. PLoS Med 2017;14(8):e1002370.
49. PROSPERO. International prospective register of systematic reviews. Disponibile a: www.crd.york.ac.uk/prospero. Ultimo accesso: 13 novembre 2017
50. Kilsdonk E, Peute LW, Jaspers MW. Factors influencing implementation success of guideline-based clinical decision support systems: A systematic review and gaps analysis. Int J Med Inform. 2017;98:56-64.
2. Djulbegovic B, Guyatt GH. Progress in evidence-based medicine: a quarter century on. Lancet 2017;390:415-423.
3. Ioannidis JPA. Hijacked evidence-based medicine: stay the course and throw the pirates overboard. J Clin Epidemiol 2017;84:11-13.
4. Greenhalgh T, Howick J, Maskrey N; Evidence Based Medicine Renaissance Group. Evidence based medicine: a movement in crisis? BMJ 2014;348:g3725.
5. Heneghan C, Mahtani KR, Goldacre B, Godlee F, Macdonald H, Jarvies D. Evidence based medicine manifesto for better healthcare. BMJ. 2017 Jun 20;357:j2973.
6. 8th International Conference for EBHC Teachers and Developers. The ecosystem of evidence: connecting generation, synthesys and translation. Disponibile a: www.ebhc.org. Ultimo accesso: 13 novembre 2017
7. Cartabellotta A. Ridurre gli sprechi e premiare il rigore scientifico nella ricerca biomedica: la campagna Lancet-REWARD. Evidence 2016;8(5): e1000142.
8. James Lind Alliance (JLA) Priority Setting Partnership (PSP). Disponibile a: www.jla.nihr.ac.uk/priority-setting-partnerships. Ultimo accesso: 13 novembre 2017
9. Lund H, Brunnhuber K, Juhl C, Robinson K, Leenaars M, Dorch BF, et al. Evidence-Based Research: le revisioni sistematiche devono sempre informare i nuovi studi primari. Evidence 2017;9(3): e1000164.
10. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jeric K, et al. SPIRIT Statement 2013: checklist per il protocollo dei trial clinici. Evidence 2016;8(8): e1000148 (30 agosto 2016).
11. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647.
12. Academy of Medical Sciences. Reproducibility and reliability of biomedical research: improving research practice. Symposium report, October 2015 Disponibile a: www.acmedsci.ac.uk/file-download/38190-56314fa158e14.pdf. Ultimo accesso: 13 novembre 2017
13. Chalmers I, Glasziou G, Godlee F. Tutti i trial devono essere registrati e tutti i risultati pubblicati. Evidence 2013;5(1): e1000032.
14. Organizzazione Mondiale della Sanità Rendere pubblici i risultati dei trial clinici: lo statement dell’Organizzazione Mondiale della Sanità . Evidence 2016;8(2): e1000134.
15. Laine C, Horton R, DeAngelis CD, Drazen JM, Frizelle FA, Godlee F, Haug C, HĂ©bert PC, Kotzin S, Marusic A, Sahni P, Schroeder TV, Sox HC, Van der Weyden MB, Verheugt FW. Clinical trial registration--looking back and moving ahead. Disponibile a: www.icmje.org/news-and-editorials/clincial_trial_reg_jun2007.html. Ultimo accesso: 13 novembre 2017
16. World Health Organization. International Clinical Trials Registry Platform (ICTRP). Disponibile a: www.who.int/ictrp. Ultimo accesso: 13 novembre 2017
17. Taichman DB, Sahni P, Pinborg A, Peiperl L, Laine C, James A, Hong ST, Haileamlak A, Gollogly L, Godlee F, Frizelle FA, Florenzano F, Drazen JM, Bauchner H, Baethge C, Backus J. Data Sharing Statements for Clinical Trials: A Requirement of the International Committee of Medical Journal Editors. Disponibile a: www.icmje.org/news-and-editorials/data_sharing_june_2017.pdf. Ultimo accesso 13 novembre 2017
18. EQUATOR Network. Enhancing the QUAlity and Transparency Of health Research. Disponibile a: www.equator-network.org. Ultimo accesso: 13 novembre 2017
19. Nasser M, Clarke M, Chalmers I, Brurberg KG, Nykvist H, Lund H, Glasziou P. What are funders doing to minimise waste in research? Lancet 2017;389:1006-1007.
20. Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res 2014;14:89.
21. Al-Shahi Salman R, Beller E, Kagan J, Hemminki E, Phillips RS, Savulescu J, Macleod M, Wisely J, Chalmers I. Increasing value and reducing waste in biomedical research regulation and management. Lancet 2014;383:176-85.
22. Ioannidis JP, Greenland S, Hlatky MA, Khoury MJ, Macleod MR, Moher D, Schulz KF, Tibshirani R. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014;383:166-75.
23. Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, GĂĽlmezoglu AM, Howells DW, Ioannidis JP, Oliver S. How to increase value and reduce waste when research priorities are set. Lancet 2014;383:156-65.
24. Evidence-Based Medicine Data Lab, University of Oxford. TrialsTracker. Disponibile a: https://trialstracker.ebmdatalab.net. Ultimo accesso: 13 novembre 2017
25. Goldacre B, Drysdale H, Powell-Smith A, et al. The COMPare Trials Project. Disponibile a: www.COMPare-trials.org. Ultimo accesso: 13 novembre 2017
26. Mannocci A, Saulle R, Colamesta V, D’Aguanno S, Giraldi G, Maffongelli E, Meggiolaro A, Semyonov L, Unim B, La Torre G. What is the impact of reporting guidelines on Public Health journals in Europe? The case of STROBE, CONSORT and PRISMA. J Public Health (Oxf) 2015;37:737-40.
27. Cochrane Training. Guides and handbooks. Disponibile a: www.training.cochrane.org/handbooks. Ultimo accesso. 13 novembre 2017
28. Cochrane Methods GRADEing group. Disponibile a: www.methods.cochrane.org/gradeing/welcome. Ultimo accesso: 13 novembre 2017
29. Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Linee guida per il reporting di revisioni sistematiche e meta-analisi: il PRISMA Statement. Evidence 2015;7(6): e1000114
30. PRISMA Statement extensions. Disponibile a: www.prisma-statement.org/Extensions. Ultimo accesso: 13 novembre 2017
31. Ioannidis JP. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q 2016;94:485-514.
32. Cochrane Database of Systematic Reviews. Number of Cochrane reviews and protocols by issue. Disponibile a: www.cochranelibrary.com/dotAsset/5ed035c1-ae96-4139-be3a-7ca0065269a2.pdf. Ultimo accesso: 13 novembre 2017
33. Cochrane Database of Systematic Reviews. Impact factor. Disponibile a: www.cochranelibrary.com/cochrane-database-of-systematic-reviews. Ultimo accesso: 13 novembre 2017
34. CRD Databases. Changes to DARE and NHS EED. 11 May 2015. Disponibile a: www.crd.york.ac.uk/crdweb/newspage.asp. Ultimo accesso: 13 novembre 2017
35. Guidelines International Network (G-I-N). Disponibile a: www.g-i-n.net. Ultimo accesso: 13 novembre 2017
36. Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P; Board of Trustees of the Guidelines International Network. Guidelines International Network: verso standard internazionali per la produzione di linee guida. Evidence 2012;4(6): e1000022
37. Brouwers M, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, Fervers B, Graham ID, Grimshaw J, Hanna S, Littlejohns P, Makarski J, Zitzelsberger L for the AGREE Next Steps Consortium. AGREE II: Advancing guideline development, reporting and evaluation in healthcare. CMAJ 2010;182:E839-42.
38. Institute of Medicine. Clinical Practice Guidelines We Can Trust. Standards for Developing Trustworthy Clinical Practice Guidelines (CPGs). Washington (DC): National Academies Press (US); 2011.
39. Schünemann HJ, Al-Ansary LA, Forland F, Kersten S, Komulainen J, Kopp IB, Macbeth F, Phillips SM, Robbins C, van der Wees P, Qaseem A; Board of Trustees of the Guidelines International Network. Disclosure e gestione dei conflitti di interesse nelle linee guida: i princìpi del Guidelines International Network. Evidence 2016;8(3): e1000136.
40. GRADE Working Group. Disponibile a: www.gradeworkinggroup.org. Ultimo accesso: 13 novembre 2017
41. Cartabellotta A, LaganĂ AS. AGREE Reporting Checklist: uno strumento per migliorare il reporting delle linee guida. Evidence 2016;8(7): e1000146.
42. Chen Y, Yang K, Marušic A, Qaseem A, Meerpohl JJ, Flottorp S, Akl EA, Schünemann HJ, Chan ES, Falck-Ytter Y, Ahmed F, Barber S, Chen C, Zhang M, Xu B, Tian J, Song F, Shang H, Tang K, Wang Q, Norris SL; RIGHT (Reporting Items for Practice Guidelines in Healthcare) Working Group. RIGHT statement: strumento per il reporting delle linee guida per la pratica clinica. Evidence 2017;9(2): e1000161.
43. Vernooij RW, Alonso-Coello P, Brouwers M, MartĂnez GarcĂa L; CheckUp Panel. Reporting Items for Updated Clinical Guidelines: Checklist for the Reporting of Updated Guidelines (CheckUp). PLoS Med 2017;14:e1002207.
44. Cartabellotta A, Tedesco T, Pomponio G. Linee guida per la valutazione clinica e il trattamento dei pazienti con multimorbiditĂ . Evidence 2016;8(10): e1000154.
45. Glasziou P, Haynes B. The paths from research to improved health outcomes. ACP J Club 2005;142(2):A8-10.
46. Graham I, Straus S, Tetroe J. Knowledge Translation in Health Care: Moving from Evidence to Practice. John Wiley & Sons, 2013.
47. Ioannidis JP, Khoury MJ. Assessing value in biomedical research: the PQRST of appraisal and reward. JAMA 2014;312:483-4.
48. Cruz Rivera S, Kyte DG, Aiyegbusi OL, Keeley TJ, Calvert MJ. Assessing the impact of healthcare research: A systematic review of methodological frameworks. PLoS Med 2017;14(8):e1002370.
49. PROSPERO. International prospective register of systematic reviews. Disponibile a: www.crd.york.ac.uk/prospero. Ultimo accesso: 13 novembre 2017
50. Kilsdonk E, Peute LW, Jaspers MW. Factors influencing implementation success of guideline-based clinical decision support systems: A systematic review and gaps analysis. Int J Med Inform. 2017;98:56-64.