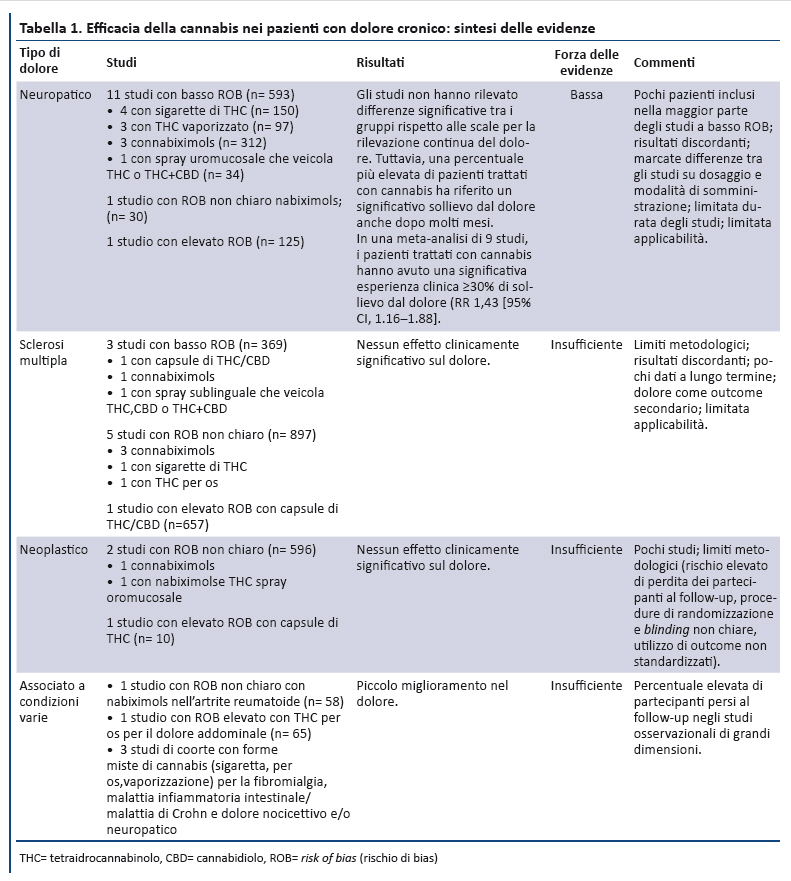
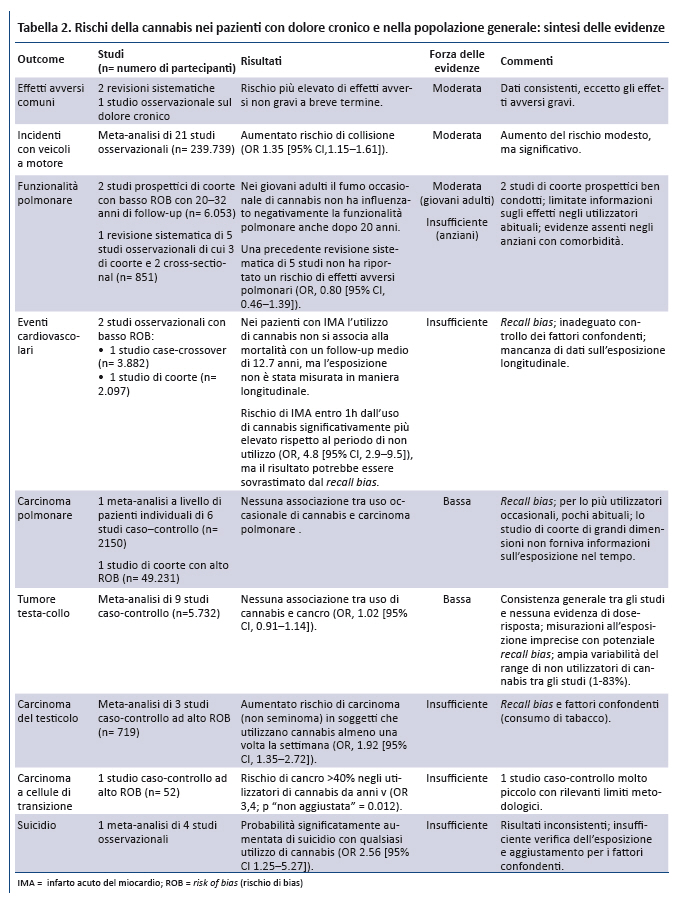
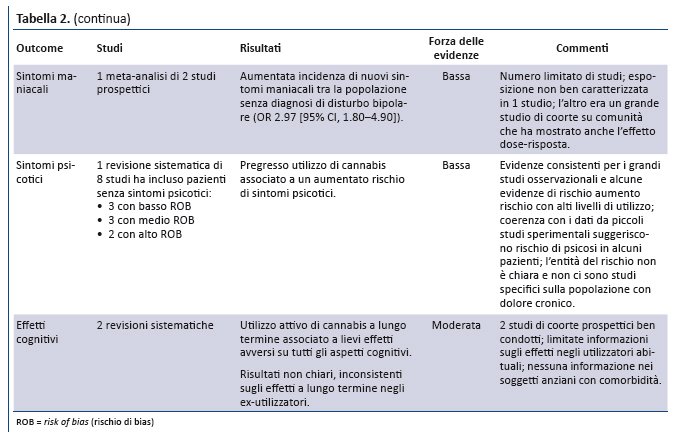
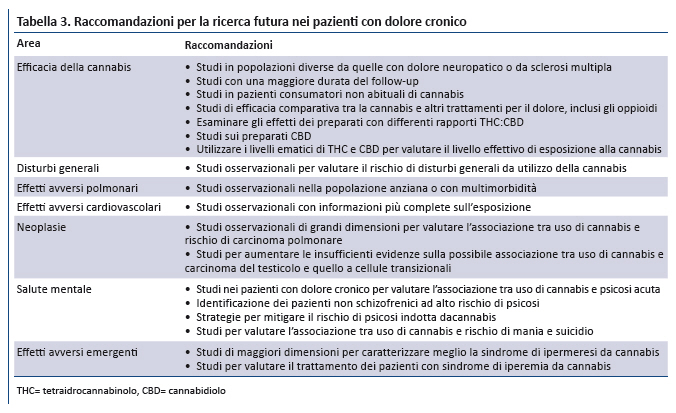
Position Statement GIMBE
Uso terapeutico della cannabis nel dolore cronico:
efficacia ed effetti avversi
Antonino Cartabellotta, Corrado IaconoEvidence 2017;9(9): e1000173 doi: 10.4470/E1000173
Pubblicato: 28 novembre 2017
Copyright: Š 2017 Cartabellotta et al. Questo è un articolo open-access, distribuito con licenza Creative Commons Attribution, che ne consente lâutilizzo, la distribuzione e la riproduzione su qualsiasi supporto esclusivamente per fini non commerciali, a condizione di riportare sempre autore e citazione originale.
1. Ryan-Ibarra S, Induni M, Ewing D. Prevalence of medical marijuana use in California, 2012. Drug Alcohol Rev 2015;34:141-6.
2. Adler JN, Colbert JA. Clinical decisions. Medicinal use of marijuanaâ polling results. N Engl J Med 2013;368:e30.
3. Legge 08 aprile 1998 , n. 94. Conversione in legge, con modificazioni, del decreto-legge 17 febbraio 1998, n. 23, recante disposizioni urgenti in materia di sperimentazioni cliniche in campo oncologico e altre misure in materia sanitaria. (G.U. Serie Generale , n. 86 del 14 aprile 1998). Disponibile a: www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=20248. Ultimo accesso: 17 novembre 2017.
4. DECRETO 23 gennaio 2013. Aggiornamento delle tabelle contenenti lâindicazione delle sostanze stupefacenti e psicotrope, di cui al decreto del Presidente della Repubblica 9 ottobre 1990, n. 309 e successive modificazioni e integrazioni. Inserimento nella Tabella II, Sezione B, dei medicinali di origine vegetale a base di Cannabis (sostanze e preparazioni vegetali, inclusi estratti e tinture). (13A00942) (GU Serie Generale n.33 del 08-02-2013). Disponibile a: www.gazzettaufficiale.it/eli/id/2013/02/08/13A00942/sg. Ultimo accesso: 17 novembre 2017.
5. DL S.2947 âDisposizioni concernenti la coltivazione e la somministrazione della cannabis a uso medicoâ. Disponibile a: www.senato.it/leg/17/BGT/Schede/Ddliter/48395.htm. Ultimo accesso: 17 novembre 2017.
6. Ministero della Salute, Ministero della Difesa. Accordo di collaborazione tra il Ministro della salute e il Ministro della Difesa per lâavvio del Progetto Pilota per la produzione nazionale di sostanze e preparazioni di origine vegetale a base di cannabis. Roma, 18 settembre 2014. Disponibile a: www.salute.gov.it/imgs/C_17_pagineAree_4588_listaFile_itemName_0_file.pdf. Ultimo accesso: 17 novembre 2017.
7. Ministero della Salute. Direzione Generale dei Dispositivi Medici e del Servizio Farmaceutico. Circolare 14/12/2016 - Inizio commercializzazione Cannabis FM-2 prodotta dallo Stabilimento Chimico Farmaceutico Militare di Firenze in attuazione dellâAccordo di collaborazione tra il Ministro della salute e il Ministro della difesa firmato in data 18 settembre 2014, concernente lâavvio del Progetto Pilota per la produzione nazionale di sostanze e preparazioni di origine vegetale a base di cannabis. Disponibile a: www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2016&codLeg=57156&parte=1%20&serie=null. Ultimo accesso: 17 novembre 2017.
8. Stabilimento Chimico Farmaceutico Militare di Firenze. Produzione cannabis ad uso medico. Disponibile a: www.farmaceuticomilitare.it/cannabis.aspx?lnrid=25. Ultimo accesso: 17 novembre 2017.
9. DECRETO 9 novembre 2015. Funzioni di Organismo statale per la cannabis previsto dagli articoli 23 e 28 della convenzione unica sugli stupefacenti del 1961, come modificata nel 1972. (15A08888) (GU Serie Generale n.279 del 30-11-2015). Disponibile a: www.gazzettaufficiale.it/eli/id/2015/11/30/15A08888/sg. Ultimo accesso: 17 novembre 2017.
10. Circolare del Ministero della Salute n. 12516 del 22 febbraio 2017. Trasmissione del documento recante raccomandazioni ai medici prescrittori di Cannabis FM-2 prodotta dallo Stabilimento Chimico Farmaceutico Militare di Firenze secondo le normative dellâUE in materia di sostanze attive, certificata GMP secondo le Goodmanufacturing practices dellâUE. Disponibile a: www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2017&codLeg=58262&parte=1%20&serie=null. Direzione Generale dei Dispositivi Medici e del Servizio Farmaceutico. Ultimo accesso: 17 novembre 2017.
11. Ministero della Salute. Medicinali a base di cannabis. Disponibile a: www.salute.gov.it/portale/temi/p2_5.jsp?lingua=italiano&area=sostanzeStupefacenti&menu=organismo. Ultimo accesso: 17 novembre 2017.
12. Nugent SM, Morasco BJ, OâNeil ME, Freeman M, Low A, Kondo K, Elven C, Zakher B, Motuâapuaka M, Paynter R, Kansagara D. The Effects of Cannabis Among Adults With Chronic Pain and an Overview of General Harms: A Systematic Review. Ann Intern Med 2017;167:319-331.
13. Bonn-Miller MO, Boden MT, Bucossi MM, Babson KA. Selfreported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alcohol Abuse 2014;40:23-30.
14. Ilgen MA, Bohnert K, Kleinberg F, Jannausch M, Bohnert AS, Walton M, et al. Characteristics of adults seeking medical marijuana certification. Drug Alcohol Depend 2013;132:654-9.
15. Degenhardt L, Lintzeris N, Campbell G, Bruno R, Cohen M, Farrell M, et al. Experience of adjunctive cannabis use for chronic noncancer pain: findings from the Pain and Opioids IN Treatment (POINT) study. Drug Alcohol Depend 2015;147:144-50.
16. Reisfield GM, Wasan AD, Jamison RN. The prevalence and significance of cannabis use in patients prescribed chronic opioid therapy: a review of the extant literature. Pain Med 2009;10:1434-41.
17. Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-73.
18. Butler M, Krebs E, Sunderlin B, Kane R. Medical cannabis for non-cancer pain: a systematic review. Disponibile a: www.health.state.mn.us/people/cannabis/docs/intractable/medicalcannabisreport.pdf. Ultimo accesso: 17 novembre 2017.
19. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10.
20. Higgins JPT, Altman DG, Sterne JAC, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; 2011. Disponibile a: www.handbook.cochrane.org. Ultimo accesso: 17 novembre 2017.
21. Wells GA, Shea B, OâConnell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Disponibile a: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Ultimo accesso: 17 novembre 2017.
22. Viswanathan M, Ansari M, Berkman N, Chang S, Hartling L, McPheeters L, et al. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. Rockville: Agency for Healthcare Research and Quality; 2012. AHRQ publication no. 12-EHC047-EF. (Methods Guide for Comparative Effectiveness Reviews). Disponibile a: www.effectivehealthcare.ahrq.gov/topics/methods-bias-update/methods/. Ultimo accesso: 17 novembre 2017
23. Berkman N, Lohr K, Ansari M, McDonagh M, Balk E, Whitlock E, et al. Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. Rockville: Agency for Healthcare Research and Quality; 2013. AHRQ publication no. 13(14)-EHC130-EF. (Methods Guide for Comparative Effectiveness Reviews). Disponibile a: www.effectivehealthcare.ahrq.gov/topics/methods-guidance-grading-evidence/methods/. Ultimo accesso: 17 novembre 2017
24. Atkins D, Chang S, Gartlehner G, Buckley D, Whitlock E, Berliner E, et al. Assessing the Applicability of Studies When Comparing Medical Interventions. Rockville: Agency for Healthcare Research and Quality; 2013. AHRQ publication no. 11-EHC019-EF. (Methods Guide for Comparative Effectiveness Reviews). Disponibile a: www.effectivehealthcare.ahrq.gov/topics/methods-guidance-applicability/methods/. Ultimo accesso: 17 novembre 2017.
25. Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 2006;45:50-2.
26. Collin C, Ehler E, Waberzinek G, Alsindi Z, Davies P, Powell K, et al. A double-blind, randomized, placebo-controlled, parallel group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010;32:451-9.
27. Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebocontrolled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010;39:167-79.
28. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy induced neuropathic pain. J Pain Symptom Manage 2014;47:166-73.
29. Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebocontrolled, graded-dose trial. J Pain 2012;13:438-49.
30. Selvarajah D, Gandhi R, Emery CJ, Tesfaye S. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: depression is a major confounding factor. Diabetes Care 2010;33:128-30.
31. Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, Lauder H, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014;18:999-1012.
32. Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008;9:506-21.
33. Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG; MUSEC Research Group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J NeurolNeurosurg Psychiatry 2012;83:1125-32.
34. Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain 2004;112:299-306.
35. Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009;34:672-80.
36. Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, et al; UK MS Research Group. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentrerandomised placebo-controlled trial. Lancet 2003;362:1517-26.
37. Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology 2007;68:515-21.
38. Noyes R Jr, Brunk SF, Avery DA, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. ClinPharmacolTher 1975;18:84-9.
39. Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain 2007;133:210-20.
40. Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of Inhaled Cannabis on Painful Diabetic Neuropathy. J Pain 2015;16:616-27.
41. Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ 2012;184:1143-50.
42. Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. MultScler 2004;10:434-41.
43. Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010;182:E694-701.
44. Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-48.
45. Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol 2013;260:984-97.
46. Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005;65:812-9.
47. Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 âN of 1â studies. Anaesthesia 2004;59:440-52.
48. Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. ClinRehabil. 2003;17:21-9.
49. van Amerongen G, Kanhai K, Baakman AC, Heuberger J, Klaassen E, Beumer TL, et al. Effects on spasticity and neuropathic pain of an oral formulation of ?9-tetrahydrocannabinol in patients with progressive multiple sclerosis. ClinTher 2017 Feb 9. pii: S0149-2918(17)30054-1.
50. de Vries M, van Rijckevorsel DC, Vissers KC, Wilder-Smith OH, van Goor H; Pain and Nociception Neuroscience Research Group. Tetrahydrocannabinol does not reduce pain in patients with chronic abdominal pain in a phase 2 placebo-controlled study. ClinGastroenterolHepatol 2017 Jul;15(7):1079-1086.e4.
51. Wilsey B, Marcotte TD, Deutsch R, Zhao H, Prasad H, Phan A. An exploratory human laboratory experiment evaluating vaporized cannabis in the treatment of neuropathic pain from spinal cord injury and disease. J Pain. 2016;17:982-1000.
52. Fiz J, DurĂĄn M, CapellĂ D, Carbonell J, FarrĂŠ M. Cannabis use in patients with fibromyalgia: effect on symptoms relief and healthrelated quality of life. PLoS One 2011;6:e18440.
53. Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohnâs disease. Inflamm Bowel Dis 2014;20:472-80.
54. Ware MA, Wang T, Shapiro S, Collet JP; COMPASS study team. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). J Pain 2015;16:1233-42.
55. Hancox RJ, Poulton R, Ely M, Welch D, Taylor DR, McLachlan CR, et al. Effects of cannabis on lung function: a population-based cohort study. EurRespir J 2010;35:42-7.
56. Pletcher MJ, Vittinghoff E, Kalhan R, Richman J, Safford M, Sidney S, et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA 2012;307:173-81.
57. Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med 2007;167:221-8.
58. Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation 2001;103:2805-9.
59. Frost L, Mostofsky E, Rosenbloom JI, Mukamal KJ, Mittleman MA. Marijuana use and long-term mortality among survivors of acute myocardial infarction. Am Heart J 2013;165:170-5.
60. de Carvalho MF, Dourado MR, Fernandes IB, Arau´ jo CT, Mesquita AT, Ramos-Jorge ML. Head and neck cancer among marijuana users: a meta-analysis of matched case-control studies. Arch Oral Biol 2015;60:1750-5.
61. Callaghan RC, Allebeck P, Sidorchuk A. Marijuana use and risk of lung cancer: a 40-year cohort study. Cancer Causes Control. 2013;24:1811-20.
62. Zhang LR, Morgenstern H, Greenland S, Chang SC, Lazarus P, Teare MD, et al; Cannabis and Respiratory Disease Research Group of New Zealand. Cannabis smoking and lung cancer risk: pooled analysis in the International Lung Cancer Consortium. Int J Cancer 2015;136:894-903.
63. Gurney J, Shaw C, Stanley J, Signal V, Sarfati D. Cannabis exposure and risk of testicular cancer: a systematic review and metaanalysis. BMC Cancer 2015;15:897.
64. Chacko JA, Heiner JG, Siu W, Macy M, Terris MK. Association between marijuana use and transitional cell carcinoma. Urology 2006;67:100-4.
65. Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007;370:319-28.
66. Kuepper R, van Os J, Lieb R, Wittchen HU, Ho¨ fler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ 2011;342:d738.
67. Dominguez MD, Saka MC, can Saka M, Lieb R, Wittchen HU, van Os J. Early expression of negative/disorganized symptoms predicting psychotic experiences and subsequent clinical psychosis: a 10-year study. Am J Psychiatry 2010;167:1075-82.
68. RĂśssler W, Hengartner MP, Angst J, Ajdacic-Gross V. Linking substance use with symptoms of subclinical psychosis in a community cohort over 30 years. Addiction 2012;107:1174-84.
69. Kaufmann RM, Kraft B, Frey R, Winkler D, Weiszenbichler S, Bäcker C, et al. Acute psychotropic effects of oral cannabis extract with a defined content of Delta9-tetrahydrocannabinol (THC) in healthy volunteers. Pharmacopsychiatry 2010;43:24-32.
70. Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol 2013;27:19-27.
71. Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195:488-91.
72. van Nierop M, Janssens M, Bruggeman R, Cahn W, de Haan L, Kahn RS, et al; Genetic Risk OUtcome of Psychosis Investigators. Evidence that transition from health to psychotic disorder can be traced to semi-ubiquitous environmental effects operating against background genetic risk. PLoS One 2013;8:e76690.
73. Mason O, Morgan CJ, Dhiman SK, Patel A, Parti N, Patel A, et al. Acute cannabis use causes increased psychotomimetic experiences in individuals prone to psychosis. Psychol Med 2009;39:951-6.
74. Gibbs M, Winsper C, Marwaha S, Gilbert E, Broome M, Singh SP. Cannabis use and mania symptoms: a systematic review and metaanalysis. J Affect Disord 2015;171:39-47.
75. Schreiner AM, Dunn ME. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. ExpClinPsychopharmacol 2012;20:420-9.
76. Ganzer F, BrĂśning S, Kraft S, Sack PM, Thomasius R. Weighing the evidence: a systematic review on long-term neurocognitive effects of cannabis use in abstinent adolescents and adults. Neuropsychol Rev 2016;26:186-222.
77. Borges G, Bagge CL, Orozco R. A literature review and metaanalyses of cannabis use and suicidality. J Affect Disord 2016;195:63-74.
78. Rogeberg O, Elvik R. The effects of cannabis intoxication on motor vehicle collision revisited and revised. Addiction 2016;111:1348-59.
79. Ramos S, Rodrigues R, Almeida N, SĂĄ JM, Fonseca L. Cannabinoid hyperemesis syndrome [Abstract]. PsychotherPsychosom2013;82(Suppl 1):90. Abstract no. 357.
80. Sadiq M. Cannabis hyperemesis syndrome [Abstract]. J Addict Med 2013;7(Suppl):E3.
81. Soriano-Co M, Batke M, Cappell MS. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: a report of eight cases in the United States. Dig Dis Sci 2010;55:3113-9.
82. Velasco A, Pentecost P. An unexpected etiology of cyclical vomiting [Abstract]. J Hosp Med 2012;7(suppl2). Abstract no. 97987.
83. Vujasinovic M, Ivartnik M, Tretjak M. Cannabinoid hyperemesissyndrome - case report. Zdravnis?kivestnik 2012;81:159-62.
84. Welder JD. Some like it hot: a case of cannabinoid hyperemesis syndrome [Abstract]. J Gen Intern Med 2012;27:S480-1.
85. Woods JA, Wright NJ, Gee J, Scobey MW. Cannabinoid hyperemesis syndrome: an emerging drug-induced disease. Am J Ther 2016;23:e601-5.
86. Sorensen CJ, DeSanto K, Borgelt L, Phillips KT, Monte AA. Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatmentâa systematic review. J Med Toxicol 2017;13:71-87.
87. Cescon DW, Page AV, Richardson S, Moore MJ, Boerner S, Gold WL. Invasive pulmonary aspergillosis associated with marijuana use in a man with colorectal cancer. J ClinOncol 2008;26:2214-5.
88. Chusid MJ, Gelfand JA, Nutter C, Fauci AS. Letter: Pulmonary aspergillosis, inhalation of contaminated marijuana smoke, chronic granulomatous disease. Ann Intern Med 1975;82:682-3.
89. Marks WH, Florence L, Lieberman J, Chapman P, Howard D, Roberts P, et al. Successfully treated invasive pulmonary aspergillosis associated with smoking marijuana in a renal transplant recipient. Transplantation 1996;61:1771-4.
90. Institute of Medicine Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Pr; 2011.
91. Munckhof WJ, Konstantinos A, Wamsley M, Mortlock M, Gilpin C. A cluster of tuberculosis associated with use of a marijuana water pipe. Int J Tuberc Lung Dis 2003;7:860-5.
92. Oeltmann JE, Oren E, Haddad MB, Lake Lk, Harrington TA, Ijaz K, et al. Tuberculosis outbreak in marijuana users, Seattle, Washington, 2004. Emerg Infect Dis 2006;12:1156-9.
93. Carabellese F, Candelli C, Martinelli D, La Tegola D, Catanesi R. Cannabis use and violent behaviour: a psychiatric patients cohort study in Southern Italy. RivPsichiatr 2013;48:43-50.
94. Myerscough R, Taylor S. The effects of marijuana on human physical aggression. J PersSocPsychol 1985;49:1541-6.
95. Blanco C, Hasin DS, Wall MM, FlĂłrez-Salamanca L, Hoertel N, Wang S, et al. Cannabis use and risk of psychiatric disorders: prospective evidence from a US national longitudinal study. JAMA Psychiatry 2016;73:388-95.
96. Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD. Substance use disorders in a primary care sample receiving daily opioid therapy. J Pain 2007;8:573-82.
97. National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: National Academies Pr; 2017.
98. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic painâUnited States, 2016. MMWR Recomm Rep. 2016;65:1-49.
99. Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL. Cannabinoid-opioid interaction in chronic pain. ClinPharmacolTher 2011;90:844-51.
100. DeGeorge M, Dawson E, Woster P, Ko M, Burke L, Bronstein K. An analysis of the association between marijuana use and potential nonadherence in patients prescribed hydrocodone. Proceedings of the 2013 annual meeting of the American Academy of Pain Medicine. Fort Lauderdale, FL: 2013, April. Poster Abstract 114. Disponibile a: www.painmed.org/2013posters/poster114.pdf. Ultimo accesso: 17 novembre 2017.
101. Hefner K, Sofuoglu M, Rosenheck R. Concomitant cannabis abuse/dependence in patients treated with opioids for non-cancer pain. Am J Addict 2015;24:538-45.
102. Ashrafioun L, Bohnert KM, Jannausch M, Ilgen MA. Characteristics of substance use disorder treatment patients using medical cannabis for pain. Addict Behav 2015;42:185-8.
103. Haroutounian S, Ratz Y, Ginosar Y, Furmanov K, Saifi F, Meidan R, et al. The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain: a prospective open-label study. Clin J Pain 2016;32:1036-1043.
104. Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn- Miller MO. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA 2015;313:2491-3.
2. Adler JN, Colbert JA. Clinical decisions. Medicinal use of marijuanaâ polling results. N Engl J Med 2013;368:e30.
3. Legge 08 aprile 1998 , n. 94. Conversione in legge, con modificazioni, del decreto-legge 17 febbraio 1998, n. 23, recante disposizioni urgenti in materia di sperimentazioni cliniche in campo oncologico e altre misure in materia sanitaria. (G.U. Serie Generale , n. 86 del 14 aprile 1998). Disponibile a: www.trovanorme.salute.gov.it/norme/dettaglioAtto?id=20248. Ultimo accesso: 17 novembre 2017.
4. DECRETO 23 gennaio 2013. Aggiornamento delle tabelle contenenti lâindicazione delle sostanze stupefacenti e psicotrope, di cui al decreto del Presidente della Repubblica 9 ottobre 1990, n. 309 e successive modificazioni e integrazioni. Inserimento nella Tabella II, Sezione B, dei medicinali di origine vegetale a base di Cannabis (sostanze e preparazioni vegetali, inclusi estratti e tinture). (13A00942) (GU Serie Generale n.33 del 08-02-2013). Disponibile a: www.gazzettaufficiale.it/eli/id/2013/02/08/13A00942/sg. Ultimo accesso: 17 novembre 2017.
5. DL S.2947 âDisposizioni concernenti la coltivazione e la somministrazione della cannabis a uso medicoâ. Disponibile a: www.senato.it/leg/17/BGT/Schede/Ddliter/48395.htm. Ultimo accesso: 17 novembre 2017.
6. Ministero della Salute, Ministero della Difesa. Accordo di collaborazione tra il Ministro della salute e il Ministro della Difesa per lâavvio del Progetto Pilota per la produzione nazionale di sostanze e preparazioni di origine vegetale a base di cannabis. Roma, 18 settembre 2014. Disponibile a: www.salute.gov.it/imgs/C_17_pagineAree_4588_listaFile_itemName_0_file.pdf. Ultimo accesso: 17 novembre 2017.
7. Ministero della Salute. Direzione Generale dei Dispositivi Medici e del Servizio Farmaceutico. Circolare 14/12/2016 - Inizio commercializzazione Cannabis FM-2 prodotta dallo Stabilimento Chimico Farmaceutico Militare di Firenze in attuazione dellâAccordo di collaborazione tra il Ministro della salute e il Ministro della difesa firmato in data 18 settembre 2014, concernente lâavvio del Progetto Pilota per la produzione nazionale di sostanze e preparazioni di origine vegetale a base di cannabis. Disponibile a: www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2016&codLeg=57156&parte=1%20&serie=null. Ultimo accesso: 17 novembre 2017.
8. Stabilimento Chimico Farmaceutico Militare di Firenze. Produzione cannabis ad uso medico. Disponibile a: www.farmaceuticomilitare.it/cannabis.aspx?lnrid=25. Ultimo accesso: 17 novembre 2017.
9. DECRETO 9 novembre 2015. Funzioni di Organismo statale per la cannabis previsto dagli articoli 23 e 28 della convenzione unica sugli stupefacenti del 1961, come modificata nel 1972. (15A08888) (GU Serie Generale n.279 del 30-11-2015). Disponibile a: www.gazzettaufficiale.it/eli/id/2015/11/30/15A08888/sg. Ultimo accesso: 17 novembre 2017.
10. Circolare del Ministero della Salute n. 12516 del 22 febbraio 2017. Trasmissione del documento recante raccomandazioni ai medici prescrittori di Cannabis FM-2 prodotta dallo Stabilimento Chimico Farmaceutico Militare di Firenze secondo le normative dellâUE in materia di sostanze attive, certificata GMP secondo le Goodmanufacturing practices dellâUE. Disponibile a: www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2017&codLeg=58262&parte=1%20&serie=null. Direzione Generale dei Dispositivi Medici e del Servizio Farmaceutico. Ultimo accesso: 17 novembre 2017.
11. Ministero della Salute. Medicinali a base di cannabis. Disponibile a: www.salute.gov.it/portale/temi/p2_5.jsp?lingua=italiano&area=sostanzeStupefacenti&menu=organismo. Ultimo accesso: 17 novembre 2017.
12. Nugent SM, Morasco BJ, OâNeil ME, Freeman M, Low A, Kondo K, Elven C, Zakher B, Motuâapuaka M, Paynter R, Kansagara D. The Effects of Cannabis Among Adults With Chronic Pain and an Overview of General Harms: A Systematic Review. Ann Intern Med 2017;167:319-331.
13. Bonn-Miller MO, Boden MT, Bucossi MM, Babson KA. Selfreported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alcohol Abuse 2014;40:23-30.
14. Ilgen MA, Bohnert K, Kleinberg F, Jannausch M, Bohnert AS, Walton M, et al. Characteristics of adults seeking medical marijuana certification. Drug Alcohol Depend 2013;132:654-9.
15. Degenhardt L, Lintzeris N, Campbell G, Bruno R, Cohen M, Farrell M, et al. Experience of adjunctive cannabis use for chronic noncancer pain: findings from the Pain and Opioids IN Treatment (POINT) study. Drug Alcohol Depend 2015;147:144-50.
16. Reisfield GM, Wasan AD, Jamison RN. The prevalence and significance of cannabis use in patients prescribed chronic opioid therapy: a review of the extant literature. Pain Med 2009;10:1434-41.
17. Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-73.
18. Butler M, Krebs E, Sunderlin B, Kane R. Medical cannabis for non-cancer pain: a systematic review. Disponibile a: www.health.state.mn.us/people/cannabis/docs/intractable/medicalcannabisreport.pdf. Ultimo accesso: 17 novembre 2017.
19. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10.
20. Higgins JPT, Altman DG, Sterne JAC, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; 2011. Disponibile a: www.handbook.cochrane.org. Ultimo accesso: 17 novembre 2017.
21. Wells GA, Shea B, OâConnell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Disponibile a: www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Ultimo accesso: 17 novembre 2017.
22. Viswanathan M, Ansari M, Berkman N, Chang S, Hartling L, McPheeters L, et al. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. Rockville: Agency for Healthcare Research and Quality; 2012. AHRQ publication no. 12-EHC047-EF. (Methods Guide for Comparative Effectiveness Reviews). Disponibile a: www.effectivehealthcare.ahrq.gov/topics/methods-bias-update/methods/. Ultimo accesso: 17 novembre 2017
23. Berkman N, Lohr K, Ansari M, McDonagh M, Balk E, Whitlock E, et al. Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. Rockville: Agency for Healthcare Research and Quality; 2013. AHRQ publication no. 13(14)-EHC130-EF. (Methods Guide for Comparative Effectiveness Reviews). Disponibile a: www.effectivehealthcare.ahrq.gov/topics/methods-guidance-grading-evidence/methods/. Ultimo accesso: 17 novembre 2017
24. Atkins D, Chang S, Gartlehner G, Buckley D, Whitlock E, Berliner E, et al. Assessing the Applicability of Studies When Comparing Medical Interventions. Rockville: Agency for Healthcare Research and Quality; 2013. AHRQ publication no. 11-EHC019-EF. (Methods Guide for Comparative Effectiveness Reviews). Disponibile a: www.effectivehealthcare.ahrq.gov/topics/methods-guidance-applicability/methods/. Ultimo accesso: 17 novembre 2017.
25. Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 2006;45:50-2.
26. Collin C, Ehler E, Waberzinek G, Alsindi Z, Davies P, Powell K, et al. A double-blind, randomized, placebo-controlled, parallel group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010;32:451-9.
27. Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebocontrolled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010;39:167-79.
28. Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy induced neuropathic pain. J Pain Symptom Manage 2014;47:166-73.
29. Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebocontrolled, graded-dose trial. J Pain 2012;13:438-49.
30. Selvarajah D, Gandhi R, Emery CJ, Tesfaye S. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: depression is a major confounding factor. Diabetes Care 2010;33:128-30.
31. Serpell M, Ratcliffe S, Hovorka J, Schofield M, Taylor L, Lauder H, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain 2014;18:999-1012.
32. Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, et al. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008;9:506-21.
33. Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG; MUSEC Research Group. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. J NeurolNeurosurg Psychiatry 2012;83:1125-32.
34. Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain 2004;112:299-306.
35. Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 2009;34:672-80.
36. Zajicek J, Fox P, Sanders H, Wright D, Vickery J, Nunn A, et al; UK MS Research Group. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicentrerandomised placebo-controlled trial. Lancet 2003;362:1517-26.
37. Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology 2007;68:515-21.
38. Noyes R Jr, Brunk SF, Avery DA, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. ClinPharmacolTher 1975;18:84-9.
39. Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain 2007;133:210-20.
40. Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of Inhaled Cannabis on Painful Diabetic Neuropathy. J Pain 2015;16:616-27.
41. Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ 2012;184:1143-50.
42. Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. MultScler 2004;10:434-41.
43. Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, et al. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010;182:E694-701.
44. Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136-48.
45. Langford RM, Mares J, Novotna A, Vachova M, Novakova I, Notcutt W, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol 2013;260:984-97.
46. Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005;65:812-9.
47. Notcutt W, Price M, Miller R, Newport S, Phillips C, Simmons S, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 âN of 1â studies. Anaesthesia 2004;59:440-52.
48. Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. ClinRehabil. 2003;17:21-9.
49. van Amerongen G, Kanhai K, Baakman AC, Heuberger J, Klaassen E, Beumer TL, et al. Effects on spasticity and neuropathic pain of an oral formulation of ?9-tetrahydrocannabinol in patients with progressive multiple sclerosis. ClinTher 2017 Feb 9. pii: S0149-2918(17)30054-1.
50. de Vries M, van Rijckevorsel DC, Vissers KC, Wilder-Smith OH, van Goor H; Pain and Nociception Neuroscience Research Group. Tetrahydrocannabinol does not reduce pain in patients with chronic abdominal pain in a phase 2 placebo-controlled study. ClinGastroenterolHepatol 2017 Jul;15(7):1079-1086.e4.
51. Wilsey B, Marcotte TD, Deutsch R, Zhao H, Prasad H, Phan A. An exploratory human laboratory experiment evaluating vaporized cannabis in the treatment of neuropathic pain from spinal cord injury and disease. J Pain. 2016;17:982-1000.
52. Fiz J, DurĂĄn M, CapellĂ D, Carbonell J, FarrĂŠ M. Cannabis use in patients with fibromyalgia: effect on symptoms relief and healthrelated quality of life. PLoS One 2011;6:e18440.
53. Storr M, Devlin S, Kaplan GG, Panaccione R, Andrews CN. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohnâs disease. Inflamm Bowel Dis 2014;20:472-80.
54. Ware MA, Wang T, Shapiro S, Collet JP; COMPASS study team. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). J Pain 2015;16:1233-42.
55. Hancox RJ, Poulton R, Ely M, Welch D, Taylor DR, McLachlan CR, et al. Effects of cannabis on lung function: a population-based cohort study. EurRespir J 2010;35:42-7.
56. Pletcher MJ, Vittinghoff E, Kalhan R, Richman J, Safford M, Sidney S, et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA 2012;307:173-81.
57. Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med 2007;167:221-8.
58. Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation 2001;103:2805-9.
59. Frost L, Mostofsky E, Rosenbloom JI, Mukamal KJ, Mittleman MA. Marijuana use and long-term mortality among survivors of acute myocardial infarction. Am Heart J 2013;165:170-5.
60. de Carvalho MF, Dourado MR, Fernandes IB, Arau´ jo CT, Mesquita AT, Ramos-Jorge ML. Head and neck cancer among marijuana users: a meta-analysis of matched case-control studies. Arch Oral Biol 2015;60:1750-5.
61. Callaghan RC, Allebeck P, Sidorchuk A. Marijuana use and risk of lung cancer: a 40-year cohort study. Cancer Causes Control. 2013;24:1811-20.
62. Zhang LR, Morgenstern H, Greenland S, Chang SC, Lazarus P, Teare MD, et al; Cannabis and Respiratory Disease Research Group of New Zealand. Cannabis smoking and lung cancer risk: pooled analysis in the International Lung Cancer Consortium. Int J Cancer 2015;136:894-903.
63. Gurney J, Shaw C, Stanley J, Signal V, Sarfati D. Cannabis exposure and risk of testicular cancer: a systematic review and metaanalysis. BMC Cancer 2015;15:897.
64. Chacko JA, Heiner JG, Siu W, Macy M, Terris MK. Association between marijuana use and transitional cell carcinoma. Urology 2006;67:100-4.
65. Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007;370:319-28.
66. Kuepper R, van Os J, Lieb R, Wittchen HU, Ho¨ fler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ 2011;342:d738.
67. Dominguez MD, Saka MC, can Saka M, Lieb R, Wittchen HU, van Os J. Early expression of negative/disorganized symptoms predicting psychotic experiences and subsequent clinical psychosis: a 10-year study. Am J Psychiatry 2010;167:1075-82.
68. RĂśssler W, Hengartner MP, Angst J, Ajdacic-Gross V. Linking substance use with symptoms of subclinical psychosis in a community cohort over 30 years. Addiction 2012;107:1174-84.
69. Kaufmann RM, Kraft B, Frey R, Winkler D, Weiszenbichler S, Bäcker C, et al. Acute psychotropic effects of oral cannabis extract with a defined content of Delta9-tetrahydrocannabinol (THC) in healthy volunteers. Pharmacopsychiatry 2010;43:24-32.
70. Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol 2013;27:19-27.
71. Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195:488-91.
72. van Nierop M, Janssens M, Bruggeman R, Cahn W, de Haan L, Kahn RS, et al; Genetic Risk OUtcome of Psychosis Investigators. Evidence that transition from health to psychotic disorder can be traced to semi-ubiquitous environmental effects operating against background genetic risk. PLoS One 2013;8:e76690.
73. Mason O, Morgan CJ, Dhiman SK, Patel A, Parti N, Patel A, et al. Acute cannabis use causes increased psychotomimetic experiences in individuals prone to psychosis. Psychol Med 2009;39:951-6.
74. Gibbs M, Winsper C, Marwaha S, Gilbert E, Broome M, Singh SP. Cannabis use and mania symptoms: a systematic review and metaanalysis. J Affect Disord 2015;171:39-47.
75. Schreiner AM, Dunn ME. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. ExpClinPsychopharmacol 2012;20:420-9.
76. Ganzer F, BrĂśning S, Kraft S, Sack PM, Thomasius R. Weighing the evidence: a systematic review on long-term neurocognitive effects of cannabis use in abstinent adolescents and adults. Neuropsychol Rev 2016;26:186-222.
77. Borges G, Bagge CL, Orozco R. A literature review and metaanalyses of cannabis use and suicidality. J Affect Disord 2016;195:63-74.
78. Rogeberg O, Elvik R. The effects of cannabis intoxication on motor vehicle collision revisited and revised. Addiction 2016;111:1348-59.
79. Ramos S, Rodrigues R, Almeida N, SĂĄ JM, Fonseca L. Cannabinoid hyperemesis syndrome [Abstract]. PsychotherPsychosom2013;82(Suppl 1):90. Abstract no. 357.
80. Sadiq M. Cannabis hyperemesis syndrome [Abstract]. J Addict Med 2013;7(Suppl):E3.
81. Soriano-Co M, Batke M, Cappell MS. The cannabis hyperemesis syndrome characterized by persistent nausea and vomiting, abdominal pain, and compulsive bathing associated with chronic marijuana use: a report of eight cases in the United States. Dig Dis Sci 2010;55:3113-9.
82. Velasco A, Pentecost P. An unexpected etiology of cyclical vomiting [Abstract]. J Hosp Med 2012;7(suppl2). Abstract no. 97987.
83. Vujasinovic M, Ivartnik M, Tretjak M. Cannabinoid hyperemesissyndrome - case report. Zdravnis?kivestnik 2012;81:159-62.
84. Welder JD. Some like it hot: a case of cannabinoid hyperemesis syndrome [Abstract]. J Gen Intern Med 2012;27:S480-1.
85. Woods JA, Wright NJ, Gee J, Scobey MW. Cannabinoid hyperemesis syndrome: an emerging drug-induced disease. Am J Ther 2016;23:e601-5.
86. Sorensen CJ, DeSanto K, Borgelt L, Phillips KT, Monte AA. Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatmentâa systematic review. J Med Toxicol 2017;13:71-87.
87. Cescon DW, Page AV, Richardson S, Moore MJ, Boerner S, Gold WL. Invasive pulmonary aspergillosis associated with marijuana use in a man with colorectal cancer. J ClinOncol 2008;26:2214-5.
88. Chusid MJ, Gelfand JA, Nutter C, Fauci AS. Letter: Pulmonary aspergillosis, inhalation of contaminated marijuana smoke, chronic granulomatous disease. Ann Intern Med 1975;82:682-3.
89. Marks WH, Florence L, Lieberman J, Chapman P, Howard D, Roberts P, et al. Successfully treated invasive pulmonary aspergillosis associated with smoking marijuana in a renal transplant recipient. Transplantation 1996;61:1771-4.
90. Institute of Medicine Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Pr; 2011.
91. Munckhof WJ, Konstantinos A, Wamsley M, Mortlock M, Gilpin C. A cluster of tuberculosis associated with use of a marijuana water pipe. Int J Tuberc Lung Dis 2003;7:860-5.
92. Oeltmann JE, Oren E, Haddad MB, Lake Lk, Harrington TA, Ijaz K, et al. Tuberculosis outbreak in marijuana users, Seattle, Washington, 2004. Emerg Infect Dis 2006;12:1156-9.
93. Carabellese F, Candelli C, Martinelli D, La Tegola D, Catanesi R. Cannabis use and violent behaviour: a psychiatric patients cohort study in Southern Italy. RivPsichiatr 2013;48:43-50.
94. Myerscough R, Taylor S. The effects of marijuana on human physical aggression. J PersSocPsychol 1985;49:1541-6.
95. Blanco C, Hasin DS, Wall MM, FlĂłrez-Salamanca L, Hoertel N, Wang S, et al. Cannabis use and risk of psychiatric disorders: prospective evidence from a US national longitudinal study. JAMA Psychiatry 2016;73:388-95.
96. Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD. Substance use disorders in a primary care sample receiving daily opioid therapy. J Pain 2007;8:573-82.
97. National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: National Academies Pr; 2017.
98. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic painâUnited States, 2016. MMWR Recomm Rep. 2016;65:1-49.
99. Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL. Cannabinoid-opioid interaction in chronic pain. ClinPharmacolTher 2011;90:844-51.
100. DeGeorge M, Dawson E, Woster P, Ko M, Burke L, Bronstein K. An analysis of the association between marijuana use and potential nonadherence in patients prescribed hydrocodone. Proceedings of the 2013 annual meeting of the American Academy of Pain Medicine. Fort Lauderdale, FL: 2013, April. Poster Abstract 114. Disponibile a: www.painmed.org/2013posters/poster114.pdf. Ultimo accesso: 17 novembre 2017.
101. Hefner K, Sofuoglu M, Rosenheck R. Concomitant cannabis abuse/dependence in patients treated with opioids for non-cancer pain. Am J Addict 2015;24:538-45.
102. Ashrafioun L, Bohnert KM, Jannausch M, Ilgen MA. Characteristics of substance use disorder treatment patients using medical cannabis for pain. Addict Behav 2015;42:185-8.
103. Haroutounian S, Ratz Y, Ginosar Y, Furmanov K, Saifi F, Meidan R, et al. The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain: a prospective open-label study. Clin J Pain 2016;32:1036-1043.
104. Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn- Miller MO. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA 2015;313:2491-3.