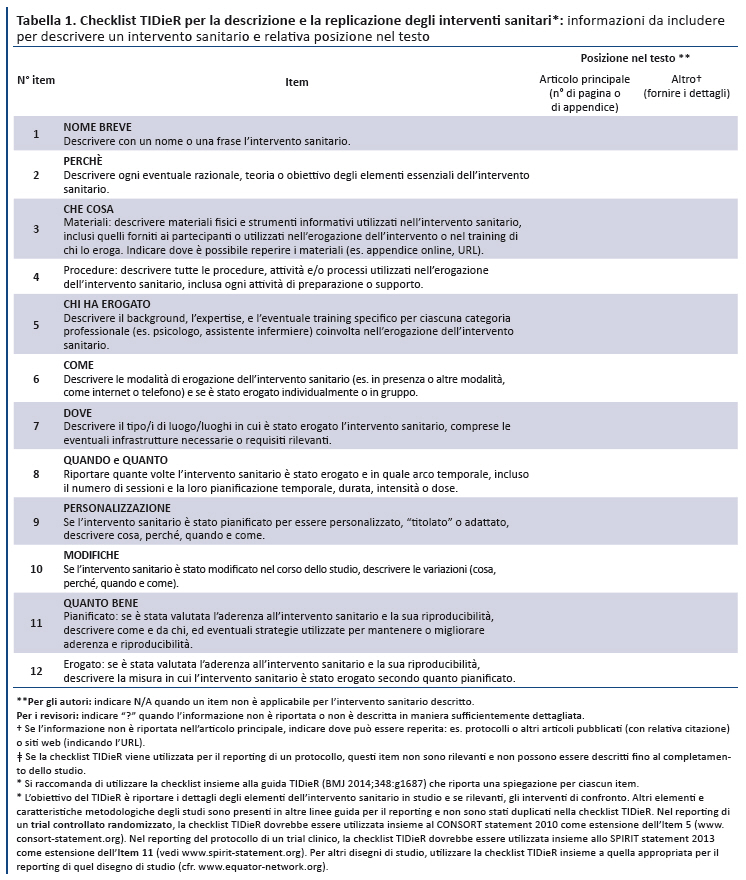
Migliorare il reporting degli interventi sanitari: la checklist TIDieR (Template for Intervention Description and Replication)
Guidelines & Standards
Migliorare il reporting degli interventi sanitari: la checklist TIDieR (Template for Intervention Description and Replication)
Tammy C Hoffmann, Paul P Glasziou, Isabelle Boutron, Ruairidh Milne, Rafael Perera, David Moher, Douglas G Altman, Virginia Barbour, Helen Macdonald, Marie Johnston, Sarah E Lamb, Mary Dixon-Woods, Peter McCulloch, Jeremy C Wyatt, An-Wen Chan, Susan MichieEvidence 2018;10(9): e1000189 doi: 10.4470/E1000189
Pubblicato: 30 ottobre 2018
Copyright: © 2018 Hoffman et al. Questo è un articolo open-access, distribuito con licenza Creative Commons Attribution, che ne consente lâutilizzo, la distribuzione e la riproduzione su qualsiasi supporto esclusivamente per fini non commerciali, a condizione di riportare sempre autore e citazione originale.
1. Duff J, Leather H, Walden E, LaPlant K, George T. Adequacy of published oncology randomised controlled trials to provide therapeutic details needed for clinical application. J Natl Cancer Inst2010;102:702-5.
2. Glasziou P, Meats E, Heneghan C, Shepperd S. What is missing from descriptions of treatment in trials and reviews? BMJ2008;336:1472-4.
3. Hoffmann T, Erueti C, Glasziou P. Poor description of non-pharmacological interventions: analysis of consecutive sample of randomised trials. BMJ2013;347:f3755.
4. Schulz K, Altman D, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ2010;340:c332.
5. Schroter S, Glasziou P, Heneghan C. Quality of descriptions of treatments: a review of published randomised controlled trials. BMJ Open2012;2:e001978.
6. Boutron I, Moher D, Altman D, Schulz K, Ravaud P. Extending the CONSORT statement to randomised trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med2008;148:295-310.
7. MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A, et al. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. PLoS Med2010;7:e1000261.
8. Gagnier J, Boon H, Rochon P, Moher D, Barnes J, Bombardier C, et al. Reporting randomised, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med2006;144:364-7.
9. Chan A, Tetzlaff J, Gøtzsche P, Altman D, Mann H, Berlin J, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ2013;346:e7586.
10. Moher D, Schulz K, Simera I, Altman D. Guidance for developers of health research reporting guidelines. PLoS Med2010;7:e1000217.
11. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014 Mar 7;348:g1687.Appendix 1: Existing checklists and literature used in generation of the Delphi survey items. Disponibile a: www.bmj.com/highwire/filestream/751837/field_highwire_adjunct_files/0/hoft015329.ww1_default.pdf. Ultimo accesso: 30 ottobre 2018
12. Murphy M, Black N, Lamping D, McKee C, Sanderson C, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess1998;2:1-88.
13. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014 Mar 7;348:g1687.Appendix 2: Main profession of Delphi survey respondents. Disponibile a: www.bmj.com/highwire/filestream/751837/field_highwire_adjunct_files/1/hoft015329.ww2_default.pdf. Ultimo accesso: 30 ottobre 2018
14. Von Elm E, Altman D, Egger M, Pocock S, Gøtzsche P, Vandenbroucke J, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ2007;335:806-8.
15. De Bruin M, Viechtbauer W, Hospers H, Schaalma H, Kok G. Standard care quality determines treatment outcomes in control groups of HAART-adherence intervention studies: implications for the interpretation and comparison of intervention effects. Health Psychol2009;28:668-74.
16. Thorpe K, Zwarenstein M, Oxman AD, Treweek S, Furberg C, Altman D, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol2009;62:464-75.
17. Gallagher LTQ, Hill C, Keamy Jr DG, Williams M, Hansen M, Maurer R, et al. Perioperative dexamethasone administration and risk of bleeding following tonsillectomy in children: a randomized controlled trial. JAMA 2013;308:1221-6.
18. Chalder M, Wiles NJ, Campbell J, Hollinghurst SP, Haase AM, Taylor AH, et al. Facilitated physical activity as a treatment for depressed adults: randomised controlled trial. BMJ 2012;344:e2758.
19. Vernooij JWP, Kaasjager HAH, van der Graaf Y, Wierdsma J, Grandjean HMH, Hovens MMC, et al. Internet based vascular risk factor management for patients with clinically manifest vascular disease: randomised controlled trial. BMJ 2012;344:e3750.
20. De Gans JD, van de Beek D. Dexamethasone in adults with bacterial meningitis. N Engl J Med 2002;347:1549-56.
21. Cromheecke ME, Levi M, Colly LP, de Mol BJ, Prins MH, Hutten BA, et al. Oral anticoagulation self-management and management by a specialist anticoagulation clinic: a randomised cross-over comparison. Lancet 2000;356:97-102.
22. Hardeman W, Kinmonth AL, Michie S, Sutton S. Impact of a physical activity intervention program on cognitive predictors of behaviour among adults at risk of type 2 diabetes (ProActive randomised controlled trial). Int J Behav Nutr Phys Act 2009;6:16.
23. Bennell KL, Bowles K, Payne C, Cicuttini F, Williamson E, Forbes A, et al. Lateral wedge insoles for medial knee osteoarthritis: 12 month randomised controlled trial. BMJ 2011;342: d2912.
24. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ2008;337:a1655.
25. McCleary N, Duncan E, Stewart F, Francis J. Active ingredients are reported more often for pharmacologic than non-pharmacologic interventions: an illustrative review of reporting practices in titles and abstracts. Trials2013;14:146.
26. Michie S, West R. Behaviour change theory and evidence: a presentation to Government. Health Psychol Rev 2013;7:1-22.
27. Dixon-Woods M, Leslie M, Tarrant C, Bion J. Explaining Matching Michigan: an ethnographic study of a patient safety program. Implement Sci 2013;8:70.
28. Dixon-Woods M, Bosk C, Aveling E, Goeschel C, Pronovost P. Explaining Michigan: developing an ex post theory of a quality improvement program. Milbank Q 2011;89:167-205.
29. Bieri F, Gray DJ, Williams GM, Raso G, Li Y-S, Yuan L, et al. Health-education package to prevent worm infections in Chinese schoolchildren. N Engl J Med 2013;368:1603-12.
30. Butler CC, Simpson SA, Hood K, Cohen D, Pickles T, Spanou C, et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: a cluster randomised trial. BMJ 2013;346:f1191.
31. Ekeberg OM, Bautz-holter E, Tveita EK, Juel NG, Kvalheim S. Subacromial ultrasound guided or systemic steroid injection for rotator cuff disease: randomised double blind study. BMJ 2009;338:a3112.
32. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014 Mar 7;348:g1687.Appendix 4: Examples of different formats that can be used to describe and/or provide study intervention materials. Disponibile a: https://www.bmj.com/highwire/filestream/751837/field_highwire_adjunct_files/3/hoft015329.ww4_default.pdf.Ultimo accesso: 30 ottobre 2018
33. Koning G, Andeweg C, Keus F, van Tilburg M, van Laarhoven C, Akkersdijk W. The transrectus sheath preperitoneal mesh repair for inguinal hernia: technique, rationale, and results of the first 50 cases. Hernia 2012;16:295-9.
34. Hijazi R, Taylor D, Richardson J. Effect of topical alkane vapocoolant spray on pain with intravenous cannulation in patients in emergency departments: randomised double blind placebo controlled trial. BMJ 2009;338:b215.
35. Reix P, Aubert F, Werck-Gallois M-C, Toutain A, Mazzocchi C, Moreux N, et al. Exercise with incorporated expiratory manoeuvres was as effective as breathing techniques for airway clearance in children with cystic fibrosis: a randomised crossover trial. J Physiother 2012;58:241-7.
36. Zurovac D, Sudoi RK, Akhwale WS, Ndiritu M, Hamer DH, Rowe AK, et al. The effect of mobile phone text-message reminders on Kenyan health workersâ adherence to malaria treatment guidelines: a cluster randomised trial. Lancet 2011;378:795-803.
37. Huang SS, Septimus E, Kleinman K, Moody J, Hickok J, Avery TR, et al. Targeted versus universal decolonisation to prevent ICU infection. N Engl J Med 2013;368:2255-65.
38. Doherty T, Tabana H, Jackson D, Swanevelder S, Fox MP, Thorson A. Effect of home based HIV counselling and testing intervention in rural South Africa: cluster randomised trial. BMJ 2013;3481:1-11.
39. Tiernan J, Hind D, Watson A, Wailoo AJ, Bradburn M, Shephard N, et al. The HubBLe trial: haemorrhoidal artery ligation (HAL) versus rubber band ligation (RBL) for haemorrhoids. BMC Gastroenterol 2012;12:153.
40. Wiles N, Thomas L, Abel A, Ridgway N, Turner N, Campbell J, et al. Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet 2013;381:375-84.
41. Kaner E, Bland M, Cassidy P, Coulton S, Dale V, Deluca P, et al. Effectiveness of screening and brief alcohol intervention in primary care (SIPS trial): pragmatic cluster randomised controlled trial. BMJ 2013;346:e8501.
42. Thomas S, Thomas PW, Kersten P, Jones R, Green C, Nock A, et al. A pragmatic parallel arm multi-centre randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based fatigue management programme (FACETS) for people with multiple sclerosis. J Neurol Neurosurg Psychiatry 2013;84:1092-9.
43. Bojang K, Akor F, Conteh L, Webb E, Bittaye O, Conway DJ, et al. Two strategies for the delivery of IPTc in an area of seasonal malaria transmission in the Gambia: a randomised controlled trial. PLoS Med 2011;8:e1000409.
44. Ybarra M, Bagci Bosi A., Korchmaros J, Emri S. A text messaging-based smoking cessation program for adult smokers: randomised controlled trial. J Med Internet Res 2012;14:e172.
45. Kessler D, Lewis G, Kaur S, Wiles N, King M, Weich S, et al. Therapist-delivered internet psychotherapy for depression in primary care: a randomised controlled trial. Lancet 2009;374:628-34.
46. Chumbler NR, Quigley P, Li X, Morey M, Rose D, Sanford J, et al. Effects of telerehabilitation on physical function and disability for stroke patients: a randomised, controlled trial. Stroke 2012;43:2168-74.
47. Halterman J, Szilagyi P, Fisher S, Fagnano M, Tremblay P, Conn K, et al. Randomised controlled trial to improve care for urban children with asthma: results of the school-based asthma therapy trial. Arch Pediatr Adolesc Med 2013;165:262-8.
48. Van den Broek NR, White SA, Goodall M, Ntonya C, Kayira E, Kafulafula G, et al. The APPLe study: a randomised, community-based, placebo-controlled trial of azithromycin for the prevention of preterm birth, with meta-analysis. PLoS Med 2009;6:e1000191.
49. Cartwright M, Hirani SP, Rixon L, Beynon M, Doll H, Bower P, et al. Effect of telehealth on quality of life and psychological outcomes over 12 months (Whole Systems Demonstrator telehealth questionnaire study): nested study of patient reported outcomes in a pragmatic, cluster randomised controlled trial. BMJ 2013;346:f653.
50. Lewycka S, Mwansambo C, Rosato M, Kazembe P, Phiri T, Mganga A, et al. Effect of womenâs groups and volunteer peer counselling on rates of mortality, morbidity, and health behaviours in mothers and children in rural Malawi (MaiMwana): a factorial, cluster-randomised controlled trial. Lancet 2013;381:1721-35.
51. Murphy AW, Cupples ME, Smith SM, Byrne M, Byrne MC, Newell J. Effect of tailored practice and patient care plans on secondary prevention of heart disease in general practice: cluster randomised controlled trial. BMJ 2009;339:b4220.
52. Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23-32.
53. Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet 2011;378:49-55.
54. McDermott MM, Ades P, Guralnik JM, Nelson M, Horn L Van, Garside D, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication. JAMA 2009;301:165-74.
55. Morrell CJ, Slade P, Warner R, Paley G, Dixon S, Walters SJ, et al. Clinical effectiveness of health visitor training in psychologically informed approaches for depression in postnatal women: pragmatic cluster randomised trial in primary care. BMJ 2009;338:a3045.
56. Stewart S, Carrington MJ, Swemmer CH, Anderson C, Kurstjens NP, Amerena J, et al. Effect of intensive structured care on individual blood pressure targets in primary care: multicentre randomised controlled trial. BMJ 2012;345:e7156.
57. Lee W-J, Wang W, Lee Y-C, Huang M-T, Ser K-H, Chen J-C. Laparoscopic mini-gastric bypass: experience with tailored bypass limb according to body weight. Obes Surg 2008;18:294-9.
58. Davidson KW, Rieckmann N, Clemow L, Schwartz JE, Shimbo D. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms. JAMA Intern Med 2010;170:600-8.
59. Wake M, Lycett K, Clifford SA, Sabin MA, Gunn J, Gibbons K, et al. Shared care obesity management in 3-10 year old children: 12 month outcomes of HopSCOTCH randomised trial. BMJ 2013;346:f3092.
60. Kapiteijn E, Marijnen C, Nagtegaal I, Putter H, Steup W, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46.
61. Morrison AP, French P, Stewart SLK, Birchwood M, Fowler D, Gumley AI, et al. Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ 2012;344:e2233.
62. Thomas KS, Crook AM, Nunn AJ, Foster K, Mason JM, Chalmers JR, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med 2013;368:1695-703.
63. Murphy AW, Cupples ME, Smith SM, Byrne M, Leathem C, Byrne MC. The SPHERE Study. Secondary prevention of heart disease in general practice: protocol of a randomised controlled trial of tailored practice and patient care plans with parallel qualitative, economic and policy analyses. Trials 2005;16:1-16.
64. Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci2007;2:40.
65. Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci D, Ory M, et al. Enhancing treatment fidelity in health behaviour change studies: best practices and recommendations from the NIH Behaviour Change Consortium. Health Psychol2004;23:443-51.
66. Coombes BK, Bisset L, Brooks P, Khan A, Vincenzio B. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: a randomized controlled trial. JAMA 2013;309:461-9.
67. Dodd MJ, Cho MH, Miaskowski C, Krasnoff J, Bank KA. A randomised controlled trial of home-based exercise for cancer-related fatigue in women during and after chemotherapy with or without radiation therapy. Cancer Nurs 2010;33:245-57.
68. Hardeman W, Michie S, Fanshawe T, Prevost T, McLoughlin K, Kinmonth AL. Fidelity of delivery of a physical activity intervention: predictors and consequences. Psychol Health2008;23:11-24.
69. Spillane V, Byrne M, Byrne M, Leathem C, OâMalley M, Cupples M. Monitoring treatment fidelity in a randomised controlled trial of a complex intervention. J Adv Nursing2007;60:343-52.
70. Hopewell S, Ravaud P, Baron G, Boutron I. Effect of editorsâ implementation of CONSORT guidelines on the reporting of abstracts in high impact medical journals: interrupted time series analysis. BMJ2012;344:e4178.
71. Hoffmann T, English T, Glasziou P. Reporting of interventions in randomised trials: an audit of journal Instructions to Authors. Trials2014;15:20.
72. Michie S, Fixsen D, Grimshaw JM, Eccles MP. Specifying and reporting complex behaviour change interventions: the need for a scientific method. Implement Sci2009;4:40.
73. Nature. For authors: manuscript formatting guide. Disponibile a: www.nature.com/nature/for-authors/formatting-guide#a5.3. Ultimo accesso: 30 ottobre 2018
74. Turner L, Shamseer L, Altman DG, Schulz KF, Moher D. Does use of the CONSORT Statement impact the completeness of reporting of randomised controlled trials published in medical journals? A Cochrane review. Syst Rev2012;1:60.
75. Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet2009;374:86-9.
76. Glasziou P, Altman D, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet2014;383:267-76.
2. Glasziou P, Meats E, Heneghan C, Shepperd S. What is missing from descriptions of treatment in trials and reviews? BMJ2008;336:1472-4.
3. Hoffmann T, Erueti C, Glasziou P. Poor description of non-pharmacological interventions: analysis of consecutive sample of randomised trials. BMJ2013;347:f3755.
4. Schulz K, Altman D, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ2010;340:c332.
5. Schroter S, Glasziou P, Heneghan C. Quality of descriptions of treatments: a review of published randomised controlled trials. BMJ Open2012;2:e001978.
6. Boutron I, Moher D, Altman D, Schulz K, Ravaud P. Extending the CONSORT statement to randomised trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med2008;148:295-310.
7. MacPherson H, Altman DG, Hammerschlag R, Youping L, Taixiang W, White A, et al. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. PLoS Med2010;7:e1000261.
8. Gagnier J, Boon H, Rochon P, Moher D, Barnes J, Bombardier C, et al. Reporting randomised, controlled trials of herbal interventions: an elaborated CONSORT statement. Ann Intern Med2006;144:364-7.
9. Chan A, Tetzlaff J, Gøtzsche P, Altman D, Mann H, Berlin J, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ2013;346:e7586.
10. Moher D, Schulz K, Simera I, Altman D. Guidance for developers of health research reporting guidelines. PLoS Med2010;7:e1000217.
11. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014 Mar 7;348:g1687.Appendix 1: Existing checklists and literature used in generation of the Delphi survey items. Disponibile a: www.bmj.com/highwire/filestream/751837/field_highwire_adjunct_files/0/hoft015329.ww1_default.pdf. Ultimo accesso: 30 ottobre 2018
12. Murphy M, Black N, Lamping D, McKee C, Sanderson C, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess1998;2:1-88.
13. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014 Mar 7;348:g1687.Appendix 2: Main profession of Delphi survey respondents. Disponibile a: www.bmj.com/highwire/filestream/751837/field_highwire_adjunct_files/1/hoft015329.ww2_default.pdf. Ultimo accesso: 30 ottobre 2018
14. Von Elm E, Altman D, Egger M, Pocock S, Gøtzsche P, Vandenbroucke J, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ2007;335:806-8.
15. De Bruin M, Viechtbauer W, Hospers H, Schaalma H, Kok G. Standard care quality determines treatment outcomes in control groups of HAART-adherence intervention studies: implications for the interpretation and comparison of intervention effects. Health Psychol2009;28:668-74.
16. Thorpe K, Zwarenstein M, Oxman AD, Treweek S, Furberg C, Altman D, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol2009;62:464-75.
17. Gallagher LTQ, Hill C, Keamy Jr DG, Williams M, Hansen M, Maurer R, et al. Perioperative dexamethasone administration and risk of bleeding following tonsillectomy in children: a randomized controlled trial. JAMA 2013;308:1221-6.
18. Chalder M, Wiles NJ, Campbell J, Hollinghurst SP, Haase AM, Taylor AH, et al. Facilitated physical activity as a treatment for depressed adults: randomised controlled trial. BMJ 2012;344:e2758.
19. Vernooij JWP, Kaasjager HAH, van der Graaf Y, Wierdsma J, Grandjean HMH, Hovens MMC, et al. Internet based vascular risk factor management for patients with clinically manifest vascular disease: randomised controlled trial. BMJ 2012;344:e3750.
20. De Gans JD, van de Beek D. Dexamethasone in adults with bacterial meningitis. N Engl J Med 2002;347:1549-56.
21. Cromheecke ME, Levi M, Colly LP, de Mol BJ, Prins MH, Hutten BA, et al. Oral anticoagulation self-management and management by a specialist anticoagulation clinic: a randomised cross-over comparison. Lancet 2000;356:97-102.
22. Hardeman W, Kinmonth AL, Michie S, Sutton S. Impact of a physical activity intervention program on cognitive predictors of behaviour among adults at risk of type 2 diabetes (ProActive randomised controlled trial). Int J Behav Nutr Phys Act 2009;6:16.
23. Bennell KL, Bowles K, Payne C, Cicuttini F, Williamson E, Forbes A, et al. Lateral wedge insoles for medial knee osteoarthritis: 12 month randomised controlled trial. BMJ 2011;342: d2912.
24. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ2008;337:a1655.
25. McCleary N, Duncan E, Stewart F, Francis J. Active ingredients are reported more often for pharmacologic than non-pharmacologic interventions: an illustrative review of reporting practices in titles and abstracts. Trials2013;14:146.
26. Michie S, West R. Behaviour change theory and evidence: a presentation to Government. Health Psychol Rev 2013;7:1-22.
27. Dixon-Woods M, Leslie M, Tarrant C, Bion J. Explaining Matching Michigan: an ethnographic study of a patient safety program. Implement Sci 2013;8:70.
28. Dixon-Woods M, Bosk C, Aveling E, Goeschel C, Pronovost P. Explaining Michigan: developing an ex post theory of a quality improvement program. Milbank Q 2011;89:167-205.
29. Bieri F, Gray DJ, Williams GM, Raso G, Li Y-S, Yuan L, et al. Health-education package to prevent worm infections in Chinese schoolchildren. N Engl J Med 2013;368:1603-12.
30. Butler CC, Simpson SA, Hood K, Cohen D, Pickles T, Spanou C, et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: a cluster randomised trial. BMJ 2013;346:f1191.
31. Ekeberg OM, Bautz-holter E, Tveita EK, Juel NG, Kvalheim S. Subacromial ultrasound guided or systemic steroid injection for rotator cuff disease: randomised double blind study. BMJ 2009;338:a3112.
32. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014 Mar 7;348:g1687.Appendix 4: Examples of different formats that can be used to describe and/or provide study intervention materials. Disponibile a: https://www.bmj.com/highwire/filestream/751837/field_highwire_adjunct_files/3/hoft015329.ww4_default.pdf.Ultimo accesso: 30 ottobre 2018
33. Koning G, Andeweg C, Keus F, van Tilburg M, van Laarhoven C, Akkersdijk W. The transrectus sheath preperitoneal mesh repair for inguinal hernia: technique, rationale, and results of the first 50 cases. Hernia 2012;16:295-9.
34. Hijazi R, Taylor D, Richardson J. Effect of topical alkane vapocoolant spray on pain with intravenous cannulation in patients in emergency departments: randomised double blind placebo controlled trial. BMJ 2009;338:b215.
35. Reix P, Aubert F, Werck-Gallois M-C, Toutain A, Mazzocchi C, Moreux N, et al. Exercise with incorporated expiratory manoeuvres was as effective as breathing techniques for airway clearance in children with cystic fibrosis: a randomised crossover trial. J Physiother 2012;58:241-7.
36. Zurovac D, Sudoi RK, Akhwale WS, Ndiritu M, Hamer DH, Rowe AK, et al. The effect of mobile phone text-message reminders on Kenyan health workersâ adherence to malaria treatment guidelines: a cluster randomised trial. Lancet 2011;378:795-803.
37. Huang SS, Septimus E, Kleinman K, Moody J, Hickok J, Avery TR, et al. Targeted versus universal decolonisation to prevent ICU infection. N Engl J Med 2013;368:2255-65.
38. Doherty T, Tabana H, Jackson D, Swanevelder S, Fox MP, Thorson A. Effect of home based HIV counselling and testing intervention in rural South Africa: cluster randomised trial. BMJ 2013;3481:1-11.
39. Tiernan J, Hind D, Watson A, Wailoo AJ, Bradburn M, Shephard N, et al. The HubBLe trial: haemorrhoidal artery ligation (HAL) versus rubber band ligation (RBL) for haemorrhoids. BMC Gastroenterol 2012;12:153.
40. Wiles N, Thomas L, Abel A, Ridgway N, Turner N, Campbell J, et al. Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet 2013;381:375-84.
41. Kaner E, Bland M, Cassidy P, Coulton S, Dale V, Deluca P, et al. Effectiveness of screening and brief alcohol intervention in primary care (SIPS trial): pragmatic cluster randomised controlled trial. BMJ 2013;346:e8501.
42. Thomas S, Thomas PW, Kersten P, Jones R, Green C, Nock A, et al. A pragmatic parallel arm multi-centre randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based fatigue management programme (FACETS) for people with multiple sclerosis. J Neurol Neurosurg Psychiatry 2013;84:1092-9.
43. Bojang K, Akor F, Conteh L, Webb E, Bittaye O, Conway DJ, et al. Two strategies for the delivery of IPTc in an area of seasonal malaria transmission in the Gambia: a randomised controlled trial. PLoS Med 2011;8:e1000409.
44. Ybarra M, Bagci Bosi A., Korchmaros J, Emri S. A text messaging-based smoking cessation program for adult smokers: randomised controlled trial. J Med Internet Res 2012;14:e172.
45. Kessler D, Lewis G, Kaur S, Wiles N, King M, Weich S, et al. Therapist-delivered internet psychotherapy for depression in primary care: a randomised controlled trial. Lancet 2009;374:628-34.
46. Chumbler NR, Quigley P, Li X, Morey M, Rose D, Sanford J, et al. Effects of telerehabilitation on physical function and disability for stroke patients: a randomised, controlled trial. Stroke 2012;43:2168-74.
47. Halterman J, Szilagyi P, Fisher S, Fagnano M, Tremblay P, Conn K, et al. Randomised controlled trial to improve care for urban children with asthma: results of the school-based asthma therapy trial. Arch Pediatr Adolesc Med 2013;165:262-8.
48. Van den Broek NR, White SA, Goodall M, Ntonya C, Kayira E, Kafulafula G, et al. The APPLe study: a randomised, community-based, placebo-controlled trial of azithromycin for the prevention of preterm birth, with meta-analysis. PLoS Med 2009;6:e1000191.
49. Cartwright M, Hirani SP, Rixon L, Beynon M, Doll H, Bower P, et al. Effect of telehealth on quality of life and psychological outcomes over 12 months (Whole Systems Demonstrator telehealth questionnaire study): nested study of patient reported outcomes in a pragmatic, cluster randomised controlled trial. BMJ 2013;346:f653.
50. Lewycka S, Mwansambo C, Rosato M, Kazembe P, Phiri T, Mganga A, et al. Effect of womenâs groups and volunteer peer counselling on rates of mortality, morbidity, and health behaviours in mothers and children in rural Malawi (MaiMwana): a factorial, cluster-randomised controlled trial. Lancet 2013;381:1721-35.
51. Murphy AW, Cupples ME, Smith SM, Byrne M, Byrne MC, Newell J. Effect of tailored practice and patient care plans on secondary prevention of heart disease in general practice: cluster randomised controlled trial. BMJ 2009;339:b4220.
52. Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23-32.
53. Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet 2011;378:49-55.
54. McDermott MM, Ades P, Guralnik JM, Nelson M, Horn L Van, Garside D, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication. JAMA 2009;301:165-74.
55. Morrell CJ, Slade P, Warner R, Paley G, Dixon S, Walters SJ, et al. Clinical effectiveness of health visitor training in psychologically informed approaches for depression in postnatal women: pragmatic cluster randomised trial in primary care. BMJ 2009;338:a3045.
56. Stewart S, Carrington MJ, Swemmer CH, Anderson C, Kurstjens NP, Amerena J, et al. Effect of intensive structured care on individual blood pressure targets in primary care: multicentre randomised controlled trial. BMJ 2012;345:e7156.
57. Lee W-J, Wang W, Lee Y-C, Huang M-T, Ser K-H, Chen J-C. Laparoscopic mini-gastric bypass: experience with tailored bypass limb according to body weight. Obes Surg 2008;18:294-9.
58. Davidson KW, Rieckmann N, Clemow L, Schwartz JE, Shimbo D. Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms. JAMA Intern Med 2010;170:600-8.
59. Wake M, Lycett K, Clifford SA, Sabin MA, Gunn J, Gibbons K, et al. Shared care obesity management in 3-10 year old children: 12 month outcomes of HopSCOTCH randomised trial. BMJ 2013;346:f3092.
60. Kapiteijn E, Marijnen C, Nagtegaal I, Putter H, Steup W, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46.
61. Morrison AP, French P, Stewart SLK, Birchwood M, Fowler D, Gumley AI, et al. Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ 2012;344:e2233.
62. Thomas KS, Crook AM, Nunn AJ, Foster K, Mason JM, Chalmers JR, et al. Penicillin to prevent recurrent leg cellulitis. N Engl J Med 2013;368:1695-703.
63. Murphy AW, Cupples ME, Smith SM, Byrne M, Leathem C, Byrne MC. The SPHERE Study. Secondary prevention of heart disease in general practice: protocol of a randomised controlled trial of tailored practice and patient care plans with parallel qualitative, economic and policy analyses. Trials 2005;16:1-16.
64. Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci2007;2:40.
65. Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci D, Ory M, et al. Enhancing treatment fidelity in health behaviour change studies: best practices and recommendations from the NIH Behaviour Change Consortium. Health Psychol2004;23:443-51.
66. Coombes BK, Bisset L, Brooks P, Khan A, Vincenzio B. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: a randomized controlled trial. JAMA 2013;309:461-9.
67. Dodd MJ, Cho MH, Miaskowski C, Krasnoff J, Bank KA. A randomised controlled trial of home-based exercise for cancer-related fatigue in women during and after chemotherapy with or without radiation therapy. Cancer Nurs 2010;33:245-57.
68. Hardeman W, Michie S, Fanshawe T, Prevost T, McLoughlin K, Kinmonth AL. Fidelity of delivery of a physical activity intervention: predictors and consequences. Psychol Health2008;23:11-24.
69. Spillane V, Byrne M, Byrne M, Leathem C, OâMalley M, Cupples M. Monitoring treatment fidelity in a randomised controlled trial of a complex intervention. J Adv Nursing2007;60:343-52.
70. Hopewell S, Ravaud P, Baron G, Boutron I. Effect of editorsâ implementation of CONSORT guidelines on the reporting of abstracts in high impact medical journals: interrupted time series analysis. BMJ2012;344:e4178.
71. Hoffmann T, English T, Glasziou P. Reporting of interventions in randomised trials: an audit of journal Instructions to Authors. Trials2014;15:20.
72. Michie S, Fixsen D, Grimshaw JM, Eccles MP. Specifying and reporting complex behaviour change interventions: the need for a scientific method. Implement Sci2009;4:40.
73. Nature. For authors: manuscript formatting guide. Disponibile a: www.nature.com/nature/for-authors/formatting-guide#a5.3. Ultimo accesso: 30 ottobre 2018
74. Turner L, Shamseer L, Altman DG, Schulz KF, Moher D. Does use of the CONSORT Statement impact the completeness of reporting of randomised controlled trials published in medical journals? A Cochrane review. Syst Rev2012;1:60.
75. Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet2009;374:86-9.
76. Glasziou P, Altman D, Bossuyt P, Boutron I, Clarke M, Julious S, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet2014;383:267-76.